
When two hydrogen atoms combine to form \[{{\text{H}}_{\text{2}}}\]molecule, the system________.
A) Becomes stable and bond is covalent in nature.
B) Becomes unstable.
C) Becomes stable and bond is ionic in nature.
D) Becomes unstable and the bond is metallic in nature.
Answer
572.7k+ views
Hint: Atoms always form molecules to attain stability. The bond between them can be ionic or covalent. The bonding makes individual atoms become stable. Matter can exist in different forms i.e, solid , liquid and gas due to this chemical bonding.
Complete step by step answer:
Atoms or elements always take part in reactions to attain stability or to have a stable outer electronic configuration. A stable outer electronic configuration makes the atom more stable. Atoms give or take electrons or share electrons to form molecules. The nature of bond depends upon whether there is sharing of electrons or there is complete transfer of electrons.
The hydrogen atom has the atomic number 1 and its electronic configuration is as follows;
\[{\text{H - 1}}{{\text{s}}^{\text{1}}}\]
A fully filled s-orbital will have two electrons and the hydrogen atom is in need of one more electron. It can make the s-orbital fully filled by sharing its one electron with the one electron of another hydrogen atom and thereby both of them will have two electrons in their outermost orbital. Thus, both the atoms will become stable. This type of bond is called covalent bonds and there occurs the sharing of electrons. The bond between hydrogen atoms in an \[{{\text{H}}_{\text{2}}}\] molecule can be depicted as;
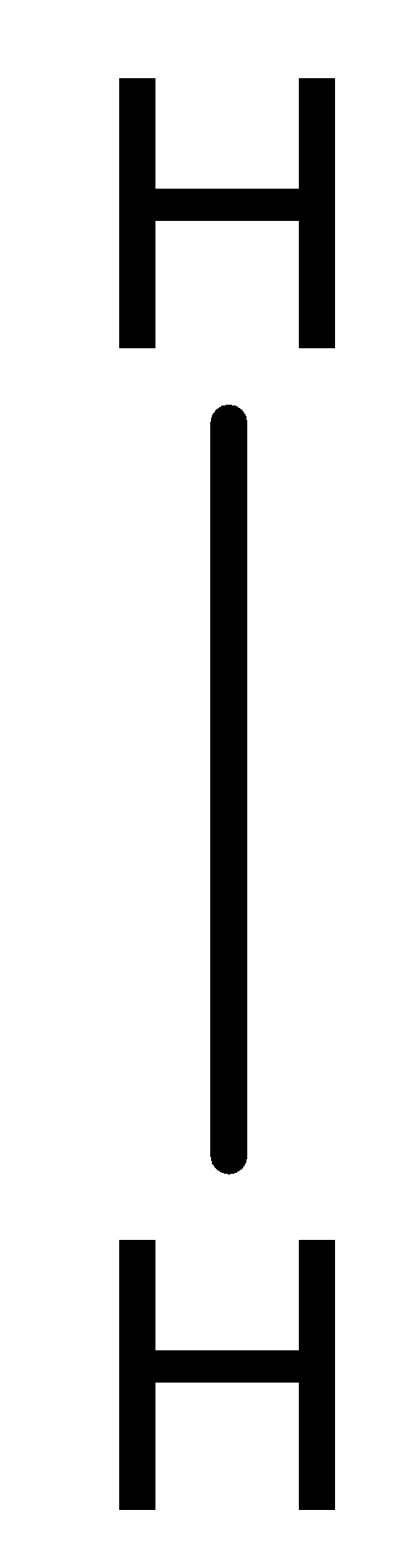
Therefore, Option A is the correct answer, that is, when two hydrogen atoms combine to form an \[{{\text{H}}_{\text{2}}}\] molecule, the system becomes stable and the bond formed is covalent in nature.
Additional information:
Ionic bonds include transfer of electrons. There will be a positive and negative ion and they combine together to form a molecule.
Note: Covalent bond formation is easier than ionic bond formation. Molecules of atoms of the same type always contain covalent bonds. But the ionic bond is always formed in between more electronegative and less electronegative atoms.
Complete step by step answer:
Atoms or elements always take part in reactions to attain stability or to have a stable outer electronic configuration. A stable outer electronic configuration makes the atom more stable. Atoms give or take electrons or share electrons to form molecules. The nature of bond depends upon whether there is sharing of electrons or there is complete transfer of electrons.
The hydrogen atom has the atomic number 1 and its electronic configuration is as follows;
\[{\text{H - 1}}{{\text{s}}^{\text{1}}}\]
A fully filled s-orbital will have two electrons and the hydrogen atom is in need of one more electron. It can make the s-orbital fully filled by sharing its one electron with the one electron of another hydrogen atom and thereby both of them will have two electrons in their outermost orbital. Thus, both the atoms will become stable. This type of bond is called covalent bonds and there occurs the sharing of electrons. The bond between hydrogen atoms in an \[{{\text{H}}_{\text{2}}}\] molecule can be depicted as;
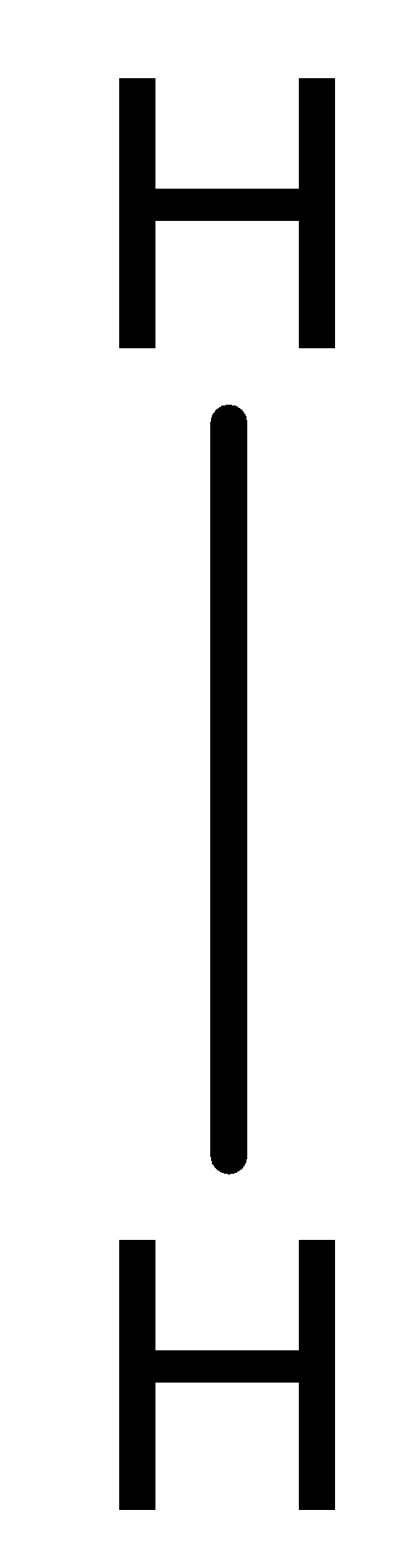
Therefore, Option A is the correct answer, that is, when two hydrogen atoms combine to form an \[{{\text{H}}_{\text{2}}}\] molecule, the system becomes stable and the bond formed is covalent in nature.
Additional information:
Ionic bonds include transfer of electrons. There will be a positive and negative ion and they combine together to form a molecule.
Note: Covalent bond formation is easier than ionic bond formation. Molecules of atoms of the same type always contain covalent bonds. But the ionic bond is always formed in between more electronegative and less electronegative atoms.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




