
Two acids have been derived from ${{H}_{2}}{{O}_{2}}$ by replacing H by ($S{{O}_{2}}OH$) group. Both the acids have one peroxy linkage
\[H-O-O-H\],\[H-O-O-S{{O}_{2}}-OH\] (${{H}_{2}}S{{O}_{5}}$)
\[H-O-O-H\],\[HO-S{{O}_{2}}-O-O-S{{O}_{2}}-OH\](${{H}_{2}}{{S}_{2}}{{O}_{8}}$)
Based on the above study, answer the following questions;
Above those 2 compounds, which is called Marshall’s acid and which is called Caro’s acid
Answer
567.9k+ views
Hint: The peroxy acids: ${{H}_{2}}S{{O}_{5}}$ is called as peroxymonosulfuric acid and ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is called as the peroxydisulfuric acid.
The names Marshall’s acid and Caro’s acid came after the names of their inventors.
Complete answer:
So this is a fact based question in which two acids are given and their bonds connectivity is also given. And we have to say that among the two acids that is derived from hydrogen peroxide, which one is called the Caro's acid and which one is called the Marshall’s acid.
So here we know that ${{H}_{2}}S{{O}_{5}}$ is also called as the peroxymonosulfuric acid, there is only one $S{{O}_{2}}OH$ group in this molecule hence the term mono is given in the nomenclature of the peroxy acid.
It was first described by Heinrich Caro and hence the peroxymonosulfuric acid (${{H}_{2}}S{{O}_{5}}$) was called the Caro’s acid.
The structure of Caro’s acid is:
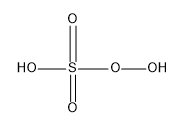
The S which is the central atom in the molecule adopts the tetrahedral geometry; the S atom here has an oxidation number of +6.
Now let’s move to the next peroxy acid ${{H}_{2}}{{S}_{2}}{{O}_{8}}$, which is called as peroxydisulfuric acid.
The molecule is called so since there are two $S{{O}_{2}}OH$ groups present in the structure of this molecule.
This acid is called Marshall's acid as it was first described by Professor Hugh Marshall, as a mark of appreciation and respect for his invention, the peroxydisulfuric acid is generally called as Marshall’s acid.
Here also the sulphur atom forms six bonds and the compound ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is commonly called as persulphates.
The structure of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is as follows
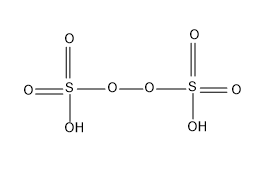
Note:
There is very high chance of getting confused between Caro’s acid (${{H}_{2}}S{{O}_{5}}$) and Marshall’s acid (${{H}_{2}}{{S}_{2}}{{O}_{8}}$) since both of them are peroxy acids and only differ by one unit of $S{{O}_{2}}OH$ group.
Both the acids are very potent oxidizing agents.
The names Marshall’s acid and Caro’s acid came after the names of their inventors.
Complete answer:
So this is a fact based question in which two acids are given and their bonds connectivity is also given. And we have to say that among the two acids that is derived from hydrogen peroxide, which one is called the Caro's acid and which one is called the Marshall’s acid.
So here we know that ${{H}_{2}}S{{O}_{5}}$ is also called as the peroxymonosulfuric acid, there is only one $S{{O}_{2}}OH$ group in this molecule hence the term mono is given in the nomenclature of the peroxy acid.
It was first described by Heinrich Caro and hence the peroxymonosulfuric acid (${{H}_{2}}S{{O}_{5}}$) was called the Caro’s acid.
The structure of Caro’s acid is:

The S which is the central atom in the molecule adopts the tetrahedral geometry; the S atom here has an oxidation number of +6.
Now let’s move to the next peroxy acid ${{H}_{2}}{{S}_{2}}{{O}_{8}}$, which is called as peroxydisulfuric acid.
The molecule is called so since there are two $S{{O}_{2}}OH$ groups present in the structure of this molecule.
This acid is called Marshall's acid as it was first described by Professor Hugh Marshall, as a mark of appreciation and respect for his invention, the peroxydisulfuric acid is generally called as Marshall’s acid.
Here also the sulphur atom forms six bonds and the compound ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is commonly called as persulphates.
The structure of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is as follows

Note:
There is very high chance of getting confused between Caro’s acid (${{H}_{2}}S{{O}_{5}}$) and Marshall’s acid (${{H}_{2}}{{S}_{2}}{{O}_{8}}$) since both of them are peroxy acids and only differ by one unit of $S{{O}_{2}}OH$ group.
Both the acids are very potent oxidizing agents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




