
Triple bond is not present in
A) Cyanogen
B) Propyne
C) Nitrous oxide
D) Nitrogen dioxide
Answer
566.7k+ views
Hint: The triple bond is the bond which contains six bonding electrons.
- Triple bonds are formed generally by certain atoms, in considering the valence electrons to get the stable configuration.
Complete Solution :
In the question four compounds are to find which of the compounds that does not possess a triple bond in their structure . For that we have to first write the formulae of each compound and then trace the structure of each structure satisfying its valency to get the best stable structure.
Now let’s trace the formulae and structures of the options given above to find out the odd one i.e. which does not have a triple bond.
Option (A)- Cyanogen
Cyanogen contains two carbon atom and two N atom and have the formulae \[{{\left( CN \right)}_{2}}\]
The carbon have a valency of four and the N has a combining capacity or valency of 3.So satisfying all the valence of the atoms we get a structure as,
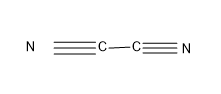
In the \[{{\left( CN \right)}_{2}}\]there are two triple bonds through which C and N are bonded, so this is not the correct option.
Option (B)- Propyne
In propyne there is C and H atoms only, the valency of C is four and that of H is 1 .The propyne have the formula ${{C}_{3}}{{H}_{4}}$, ${{C}_{n}}{{H}_{2n-2}}$
From the name of the compound itself we could say it contains a triple bond since the name end with –yne and it’s a alkyne and the formulae also supports the general formula of alkyne which is, ${{C}_{n}}{{H}_{2n-2}}$
Here n is the number of C atoms present in the skeletal chain.
The structure of propyne is:
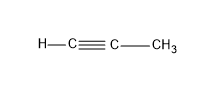
Here also as there is one triple bond, this is not the answer.
Option (C) –Nitrous oxide
In nitrous oxide there are two N atoms and one O atom, which have valencies 3 and 2 respectively. The formulae of the molecule is ${{N}_{2}}O$
The structure is as follows-
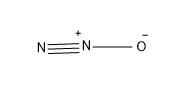
This molecule also contains a triple bond so this is not the answer.
Option (D) Nitrogen dioxide
In Nitrogen dioxide, there are two O atom and one N. The O tom have a valence of two and the N atom have a valency of 3 and the structure is as given below-
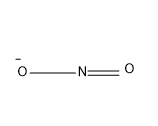
Here in this molecule there is no triple bond, so this is the correct answer.
So, the correct answer is “Option D”.
Note: The valency or the combination number of the atoms should be known so that we can easily draw the structure by satisfying the atoms through an octet formation or a charge ion which is highly stable
- Triple bonds are formed generally by certain atoms, in considering the valence electrons to get the stable configuration.
Complete Solution :
In the question four compounds are to find which of the compounds that does not possess a triple bond in their structure . For that we have to first write the formulae of each compound and then trace the structure of each structure satisfying its valency to get the best stable structure.
Now let’s trace the formulae and structures of the options given above to find out the odd one i.e. which does not have a triple bond.
Option (A)- Cyanogen
Cyanogen contains two carbon atom and two N atom and have the formulae \[{{\left( CN \right)}_{2}}\]
The carbon have a valency of four and the N has a combining capacity or valency of 3.So satisfying all the valence of the atoms we get a structure as,

In the \[{{\left( CN \right)}_{2}}\]there are two triple bonds through which C and N are bonded, so this is not the correct option.
Option (B)- Propyne
In propyne there is C and H atoms only, the valency of C is four and that of H is 1 .The propyne have the formula ${{C}_{3}}{{H}_{4}}$, ${{C}_{n}}{{H}_{2n-2}}$
From the name of the compound itself we could say it contains a triple bond since the name end with –yne and it’s a alkyne and the formulae also supports the general formula of alkyne which is, ${{C}_{n}}{{H}_{2n-2}}$
Here n is the number of C atoms present in the skeletal chain.
The structure of propyne is:
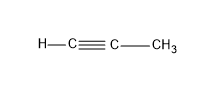
Here also as there is one triple bond, this is not the answer.
Option (C) –Nitrous oxide
In nitrous oxide there are two N atoms and one O atom, which have valencies 3 and 2 respectively. The formulae of the molecule is ${{N}_{2}}O$
The structure is as follows-
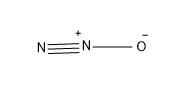
This molecule also contains a triple bond so this is not the answer.
Option (D) Nitrogen dioxide
In Nitrogen dioxide, there are two O atom and one N. The O tom have a valence of two and the N atom have a valency of 3 and the structure is as given below-

Here in this molecule there is no triple bond, so this is the correct answer.
So, the correct answer is “Option D”.
Note: The valency or the combination number of the atoms should be known so that we can easily draw the structure by satisfying the atoms through an octet formation or a charge ion which is highly stable
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




