
Total number of $\sigma $bonds in ${{N}_ {3}}H$is _______
Answer
585.9k+ views
Hint: Hydrazoic acid, also known as hydrogen azide or azoimide, is a compound with the chemical formula ${{N}_ {3}}H$. It is a colourless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride.
Complete step by step solution:
We have been provided with a compound ${{N}_ {3}}H$,
Its IUPAC name Hydrogen azide is a nitrogen hydride. It is a conjugate acid of an azide anion,
The hybridisation of the central N atom is only $sp$. And, remaining two N atoms is $s{{p}^ {2}} $
Its structure is:
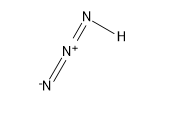
Sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
Pi bonds are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally. Each of these atomic orbitals has zero electron density at a shared nodal plane, passing through the two bonded nuclei.
From this we came to know that Hydrazoic acid has 3 sigma bonds with 3 hydrogens and zero pi bonds and has a lone pair of electrons.
Note: ${{N}_ {3}}H$ is called the covalent azide and it does not dissociate easily. This makes sodium azide more stable than hydrazoic acid. Hence, $N{{a}^ {+}} {{N}^ {3-}} $decomposes at a much higher temperature than ${{N}_ {3}}H$and hence, the higher thermal stability of $N{{H}_ {3}} Na$.
Complete step by step solution:
We have been provided with a compound ${{N}_ {3}}H$,
Its IUPAC name Hydrogen azide is a nitrogen hydride. It is a conjugate acid of an azide anion,
The hybridisation of the central N atom is only $sp$. And, remaining two N atoms is $s{{p}^ {2}} $
Its structure is:

Sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
Pi bonds are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally. Each of these atomic orbitals has zero electron density at a shared nodal plane, passing through the two bonded nuclei.
From this we came to know that Hydrazoic acid has 3 sigma bonds with 3 hydrogens and zero pi bonds and has a lone pair of electrons.
Note: ${{N}_ {3}}H$ is called the covalent azide and it does not dissociate easily. This makes sodium azide more stable than hydrazoic acid. Hence, $N{{a}^ {+}} {{N}^ {3-}} $decomposes at a much higher temperature than ${{N}_ {3}}H$and hence, the higher thermal stability of $N{{H}_ {3}} Na$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




