
What is the total number of $S - S$ bonds in polythionic acid?
(A) $n$
(B) $n - 1$
(C) $n - 2$
(D) $n - 3$
Answer
571.8k+ views
Hint:As we know that polythionic acid contains a straight chain compound of sulphur atoms having the formula ${H_2}{S_n}{O_6}$ where the number of sulphur atoms can differ in every compound formed and they are basically the conjugate acids of polythionates which are commonly available rather than polythionic acids that are rare.
Complete answer:As we know that polythionic acids are basically the conjugate acids of polythionates which are common and important rather than the polythionic acids which are rarely encountered. They contain a straight chain of sulphur atoms and are attached with two hydrogen and six oxygen atoms.
We should know that Polythionic acids have a chemical formula which is ${H_2}{S_n}{O_6}$ where the number of sulphur atoms may vary in compounds formed. The basic condition is that the number of sulphur atoms should be more than two.
For instance: ${H_2}{S_3}{O_6}$ is called the trithionic acid and the number of sulphur in this compound is three, so the $S - S$ bonds in this compound will also be $2$. Similarly we have tetrathionic acid with a chemical formula ${H_2}{S_4}{O_6}$, so it will have $3$.
So we can say that the skeletal formula of any polythionic acid can be given as:
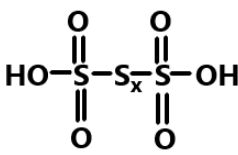
Hence, in conclusion we can say that all the polythionic acids are named through the number of atoms in the straight chain of sulphur atoms. And the basic formula to calculate the number of sulphur bonds is $n - 1$.
Therefore the correct answer is (B).
Note:Remember that a clear mechanism is not clear about the manufacturing or synthesis of polythionic acids. But, the reaction between hydrogen sulphide and sulphur dioxide in aqueous solution can yield a mixture of oxyacids of sulphur having different structure. Most stable polythionic acids are those having small numbers of sulphur atoms in the chain.
Complete answer:As we know that polythionic acids are basically the conjugate acids of polythionates which are common and important rather than the polythionic acids which are rarely encountered. They contain a straight chain of sulphur atoms and are attached with two hydrogen and six oxygen atoms.
We should know that Polythionic acids have a chemical formula which is ${H_2}{S_n}{O_6}$ where the number of sulphur atoms may vary in compounds formed. The basic condition is that the number of sulphur atoms should be more than two.
For instance: ${H_2}{S_3}{O_6}$ is called the trithionic acid and the number of sulphur in this compound is three, so the $S - S$ bonds in this compound will also be $2$. Similarly we have tetrathionic acid with a chemical formula ${H_2}{S_4}{O_6}$, so it will have $3$.
So we can say that the skeletal formula of any polythionic acid can be given as:
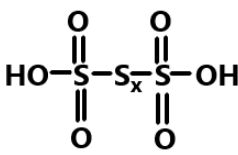
Hence, in conclusion we can say that all the polythionic acids are named through the number of atoms in the straight chain of sulphur atoms. And the basic formula to calculate the number of sulphur bonds is $n - 1$.
Therefore the correct answer is (B).
Note:Remember that a clear mechanism is not clear about the manufacturing or synthesis of polythionic acids. But, the reaction between hydrogen sulphide and sulphur dioxide in aqueous solution can yield a mixture of oxyacids of sulphur having different structure. Most stable polythionic acids are those having small numbers of sulphur atoms in the chain.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




