
What is the total number of electrons in the correct Lewis dot formula of the sulfite ion?
(A) $26$
(B) $32$
(C) $30$
(D) \[8\]
Answer
573.9k+ views
Hint:Lewis dot structure or electron dot structure show the bonding between different atoms and also represents the lone pair. The number of dots around the symbol of an element in Lewis dot structure represents the number of valence electrons.
Complete answer:
In \[1961\], Kossel and Lewis individually explained the formation of chemical bonds in terms of electrons.
Lewis made some assumptions to explain the chemical bonding:
He assumed the atom as ‘kernel’ having positive charge and the outer shells accommodate maximum eight electrons occupying corners of the cube, surrounding the ‘kernel’.
He finally concluded that the atoms form chemical bonds to obtain a stable octet
In Lewis dot structure, the electrons of valence shell or the valence shell or the valence electrons are shown around the symbol of the atom.
For example, \[Na\](sodium), have atomic number \[11\]and they have only one valence electron which can be represented as:
-Na
In the given question, we need to calculate the total number of electrons in sulfite.
Structure of sulfite is:
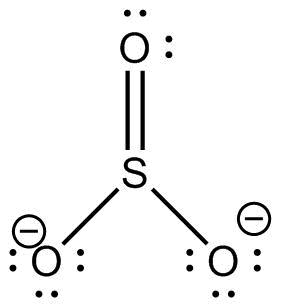
A Sulphite molecule is made up of one Sulphur and three oxygen atoms.
Total number of electrons in Sulphur \[ = 6\]
Total number of electrons in oxygen \[ = 3\left( 6 \right) = 18\]
Electrons added due to negative charge\[ = 2\]
Hence, sulphites have electrons\[ = 6 + 18 + 2 = 26\] electrons.
Note:
-For calculating the total number of electrons in a Lewis dot structure, we add both the bonded electron and lone pair of electrons.
-Also, the charge is an important factor in determining the total number of electrons. If negative charge is there on the electron which means gain of electron, then that number of electrons are added in the total number of electrons. Whereas, if the positive charge is there on the molecule, then that number of electrons are subtracted from the total number of electrons.
Complete answer:
In \[1961\], Kossel and Lewis individually explained the formation of chemical bonds in terms of electrons.
Lewis made some assumptions to explain the chemical bonding:
He assumed the atom as ‘kernel’ having positive charge and the outer shells accommodate maximum eight electrons occupying corners of the cube, surrounding the ‘kernel’.
He finally concluded that the atoms form chemical bonds to obtain a stable octet
In Lewis dot structure, the electrons of valence shell or the valence shell or the valence electrons are shown around the symbol of the atom.
For example, \[Na\](sodium), have atomic number \[11\]and they have only one valence electron which can be represented as:
-Na
In the given question, we need to calculate the total number of electrons in sulfite.
Structure of sulfite is:

A Sulphite molecule is made up of one Sulphur and three oxygen atoms.
Total number of electrons in Sulphur \[ = 6\]
Total number of electrons in oxygen \[ = 3\left( 6 \right) = 18\]
Electrons added due to negative charge\[ = 2\]
Hence, sulphites have electrons\[ = 6 + 18 + 2 = 26\] electrons.
Note:
-For calculating the total number of electrons in a Lewis dot structure, we add both the bonded electron and lone pair of electrons.
-Also, the charge is an important factor in determining the total number of electrons. If negative charge is there on the electron which means gain of electron, then that number of electrons are added in the total number of electrons. Whereas, if the positive charge is there on the molecule, then that number of electrons are subtracted from the total number of electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




