
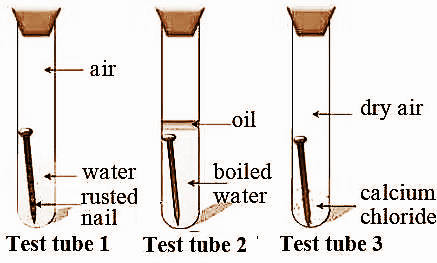
Three experiments to study the process of rusting are given below. Observe the three test tubes and answer the following questions.
a. Why is the nail in test tube 2 not rusted?
b. Why is the nail in the test tube1 rusted highly?
c. Would the nail in test tube 3 get rusted?

Answer
579.3k+ views
Hint: Rusting is an example of corrosion. The iron metal undergoes corrosion in presence of oxygen, water and the iron metal should be in contact with the oxygen and water. Rusting of iron is an example of oxidation reaction.
Complete answer:
- In the question it is given that there are three test tubes containing nails in common under different environmental conditions.
- We have to answer the given questions by observing the test tubes.
a. The nail in test tube 2 is not rusted.
- The test tube 2 contains boiled water, therefore all the dissolved oxygen is going to evaporate at the times of boiling. That means no supply of oxygen to the nail.
- So, there is a lack of oxygen in test tube 2.
- That is why the nail in test tube 2 does not get rusted.
b. The nail in test tube 1 is rusted.
- In test tube 1the nail is dipped in water, water contains some amount of dissolved oxygen.
- Generally rusting of nails happens in the presence of oxygen and water.
- In the test tube there is presence of water and oxygen then the nail in test tube 1 gets rusted.
c. The nail in test tube 3 won’t get rust.
- In test tube 3 there is a chemical called calcium chloride.
- Calcium chloride is a good absorber of oxygen.
- The calcium chloride absorbs the oxygen which is present in test tube 3.
- So, the nail which is in test tube 3 won’t get rust.
Note:
The nail is composed of iron. Iron will get rust easily in the presence of oxygen and water. The iron will lose electrons and convert into ferric hydroxide (rust) in the presence of oxygen and water (moisture).
Complete answer:
- In the question it is given that there are three test tubes containing nails in common under different environmental conditions.
- We have to answer the given questions by observing the test tubes.
a. The nail in test tube 2 is not rusted.
- The test tube 2 contains boiled water, therefore all the dissolved oxygen is going to evaporate at the times of boiling. That means no supply of oxygen to the nail.
- So, there is a lack of oxygen in test tube 2.
- That is why the nail in test tube 2 does not get rusted.
b. The nail in test tube 1 is rusted.
- In test tube 1the nail is dipped in water, water contains some amount of dissolved oxygen.
- Generally rusting of nails happens in the presence of oxygen and water.
- In the test tube there is presence of water and oxygen then the nail in test tube 1 gets rusted.
c. The nail in test tube 3 won’t get rust.
- In test tube 3 there is a chemical called calcium chloride.
- Calcium chloride is a good absorber of oxygen.
- The calcium chloride absorbs the oxygen which is present in test tube 3.
- So, the nail which is in test tube 3 won’t get rust.
Note:
The nail is composed of iron. Iron will get rust easily in the presence of oxygen and water. The iron will lose electrons and convert into ferric hydroxide (rust) in the presence of oxygen and water (moisture).
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




