
The volume-temperature graphs of a given mass of an ideal gas at constant pressure are shown below:
What is the correct order of pressures?
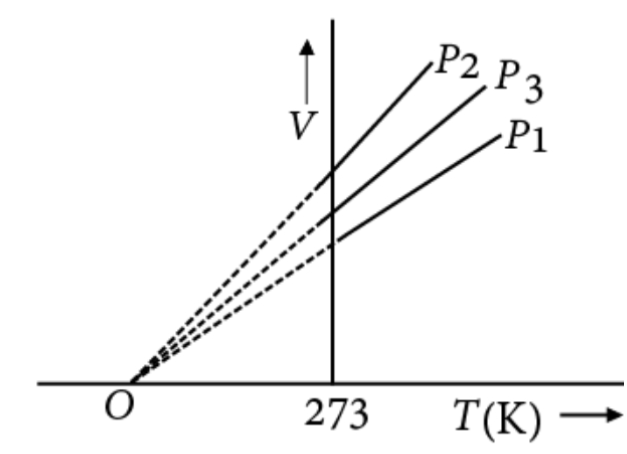
(A) $ {P_1} > {P_3} > {P_2} $
(B) $ {P_1} > {P_2} > {P_3} $
(C) $ {P_2} > {P_3} > {P_1} $
(D) $ {P_2} > {P_1} > {P_3} $

Answer
507.9k+ views
Hint :Volume is the area occupied by gas, temperature is the measurement degree of the heat whereas the pressure is the force of particular gas when put inside a container. It was sir, Emile Clapeyron who connects all the gas laws proposed by Boyle, Charles, Gay Lussac, and Avogadro and framed the equation $ PV = nRT $ . For all, P is pressure, V is volume, n is number of gases molecules, R is universal gas constant and T is temperature.
Complete Step By Step Answer:
Gaseous state of matter is one of the five other states of matter namely solids, liquids, plasma and bosons. Among solids, liquids and gases, the gaseous state of matter had attracted the scientist the most because of its ability to have minimum forces of attraction and still having such importance for the existence of life. From oxygen important for combustion to the carbon dioxide required by plants for the photo-chemical process also called photosynthesis. They all are gas. Several laws were proposed to understand the nature and behaviour of the gases like, Boyle’s law, Charles’s law, Gay Lussac law, and the Avogadro law. These were this gaseous state which failed the great kinetic theory of gases developed by Maxwell and Boltzmann.
Emile Clapeyron when connecting all the gas laws gave the ideal gas equation which is $ PV = nRT $ .
If we take pressure to be constant in the ideal gas equation, then we can write volume as $ V = \dfrac{{nRT}}{P} $ . And if we replace all the constants by one constant, let say ‘ $ K $ ’ then the equation becomes $ V = KT $ . Now according to the equation of a straight line the constant is slope, that means $ K $ is slope of the line. And $ K = \dfrac{{nR}}{P} $ that is slope is inversely proportional to the pressure. Thus, the correct order of the pressure is $ {P_1} > {P_3} > {P_2} $ .
Note :
The equation of a straight line is $ y = mx + c $ where $ x $ and $ y $ are the axises and the $ m $ and $ c $ are the constants. The constant $ c $ is the intercept that means it determines the variation of a line from being linear to being a line with a particular slope. It is to remember, that slope is always inversely proportional to the volume and pressure which is in accordance with Boyle's law as well.
Complete Step By Step Answer:
Gaseous state of matter is one of the five other states of matter namely solids, liquids, plasma and bosons. Among solids, liquids and gases, the gaseous state of matter had attracted the scientist the most because of its ability to have minimum forces of attraction and still having such importance for the existence of life. From oxygen important for combustion to the carbon dioxide required by plants for the photo-chemical process also called photosynthesis. They all are gas. Several laws were proposed to understand the nature and behaviour of the gases like, Boyle’s law, Charles’s law, Gay Lussac law, and the Avogadro law. These were this gaseous state which failed the great kinetic theory of gases developed by Maxwell and Boltzmann.
Emile Clapeyron when connecting all the gas laws gave the ideal gas equation which is $ PV = nRT $ .
If we take pressure to be constant in the ideal gas equation, then we can write volume as $ V = \dfrac{{nRT}}{P} $ . And if we replace all the constants by one constant, let say ‘ $ K $ ’ then the equation becomes $ V = KT $ . Now according to the equation of a straight line the constant is slope, that means $ K $ is slope of the line. And $ K = \dfrac{{nR}}{P} $ that is slope is inversely proportional to the pressure. Thus, the correct order of the pressure is $ {P_1} > {P_3} > {P_2} $ .
Note :
The equation of a straight line is $ y = mx + c $ where $ x $ and $ y $ are the axises and the $ m $ and $ c $ are the constants. The constant $ c $ is the intercept that means it determines the variation of a line from being linear to being a line with a particular slope. It is to remember, that slope is always inversely proportional to the volume and pressure which is in accordance with Boyle's law as well.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




