
The total number of isomers of trimethylbenzene is:
a. 2
b. 3
c. 4
d. 6
Answer
607.5k+ views
Hint: Remember that there are 6 possible locations for a methyl substituent on a benzene ring and the given compound has 3 methyl substituents. With this in mind, try to figure out the total number of possible isomers.
Complete step by step answer:
Let us look into each isomer of trimethyl benzene and its possible application to help answer the given question.
Hemimellitene, also known as 1,2,3-Trimethylbenzene, is an organic compound with the chemical formula \[{{C}_{6}}{{H}_{3}}{{\left( C{{H}_{3}} \right)}_{3}}\]. It is classified as an aromatic hydrocarbon and is physically observed to be a flammable colourless liquid. It is almost insoluble in water but is found to be soluble in organic solvents. It is found to occur naturally in coal tar and petroleum is one of the geometrical isomers of trimethylbenzene. It is mostly used in jet fuel, upon mixing with other hydrocarbons, to prevent the formation of solid particles which might cause damage to the engine of the aircraft

1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula \[{{C}_{6}}{{H}_{3}}{{\left( C{{H}_{3}} \right)}_{3}}\]. It is also classified as an aromatic hydrocarbon and it is physically observed to be a flammable colourless liquid with a strong odour. Like hemimellitene, it is also found to be nearly insoluble in water but soluble in organic solvents. Also, just like hemimellitene, pseudocumene also occurs naturally in coal tar and petroleum (about 3%). It is another one of the geometrical isomers of trimethylbenzene.

Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with the three methyl substituents positioned symmetrically around the ring and is the final geometrical isomer of trimethyl benzene. In contrast to the other geometrical isomers is a colourless liquid with sweet aromatic odour. However, like the other two geometrical isomers, it is also a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals.
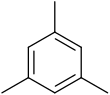
Thus, we can easily conclude that the answer to this question is b).
Note:
Mesitylene is mainly used as a precursor to 2,4,6-trimethylaniline, a precursor to colorants. This derivative is prepared by selective mononitration of mesitylene, avoiding oxidation of the methyl groups.
Complete step by step answer:
Let us look into each isomer of trimethyl benzene and its possible application to help answer the given question.
Hemimellitene, also known as 1,2,3-Trimethylbenzene, is an organic compound with the chemical formula \[{{C}_{6}}{{H}_{3}}{{\left( C{{H}_{3}} \right)}_{3}}\]. It is classified as an aromatic hydrocarbon and is physically observed to be a flammable colourless liquid. It is almost insoluble in water but is found to be soluble in organic solvents. It is found to occur naturally in coal tar and petroleum is one of the geometrical isomers of trimethylbenzene. It is mostly used in jet fuel, upon mixing with other hydrocarbons, to prevent the formation of solid particles which might cause damage to the engine of the aircraft

1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula \[{{C}_{6}}{{H}_{3}}{{\left( C{{H}_{3}} \right)}_{3}}\]. It is also classified as an aromatic hydrocarbon and it is physically observed to be a flammable colourless liquid with a strong odour. Like hemimellitene, it is also found to be nearly insoluble in water but soluble in organic solvents. Also, just like hemimellitene, pseudocumene also occurs naturally in coal tar and petroleum (about 3%). It is another one of the geometrical isomers of trimethylbenzene.

Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with the three methyl substituents positioned symmetrically around the ring and is the final geometrical isomer of trimethyl benzene. In contrast to the other geometrical isomers is a colourless liquid with sweet aromatic odour. However, like the other two geometrical isomers, it is also a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals.

Thus, we can easily conclude that the answer to this question is b).
Note:
Mesitylene is mainly used as a precursor to 2,4,6-trimethylaniline, a precursor to colorants. This derivative is prepared by selective mononitration of mesitylene, avoiding oxidation of the methyl groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




