
The total number of isomers including stereoisomers for 1,2-dimethylcyclobutane is:
A.2, one cis and one trans (both optically inactive)
B.3, one cis and two optically active trans forms
C.3, one trans and two optically active cis forms
D.4, two cis (optically active) and two trans (optically active)
Answer
572.1k+ views
Hint:Isomers are molecules which have the same number of atoms and the same molecular formula but they differ in structure and arrangements of atoms in a compound. There are various types of isomers depending on the difference in their orientation. Stereoisomers are isomers with the same molecular formula but different three dimensional orientations of the atoms.
Complete answer:
Isomers are molecules which have the same number of atoms and therefore, they have the same molecular formula. They may have the same molecular formula but they have different structure and arrangement of atoms in a compound. These isomers usually have different physical and chemical properties from each other.
Stereoisomers are types of isomers which have the same molecular formula and same number of atoms but they have different spatial arrangement of atoms that means that they have different three dimensional orientations of atoms in space. Enantiomers are optical isomers which are non-superimposable mirror images. Cis isomers and trans isomers are geometric isomers which differ in terms of position of a group of atoms in a compound.
For the above question,
1,2-dimethyl cyclobutane has one cis and two optically active trans isomers. Optically active trans isomers refer to those trans isomers which cannot be superimposed on their mirror images and are thus chiral and optically active thus, they are capable of optical rotation.
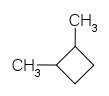
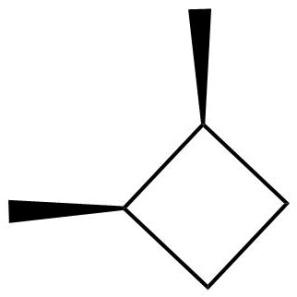
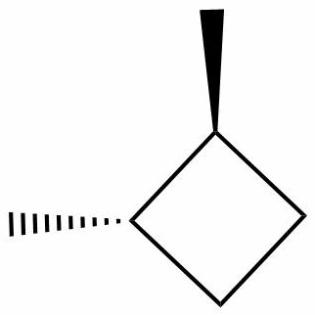
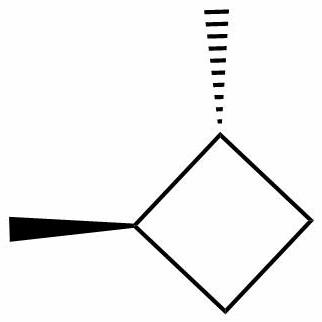
Thus, the correct option is (B) 3, has one cis and two optically active trans forms.
Note:
Stereoisomers which are non superimposable on their mirror images are known as enantiomers and a mixture containing two enantiomers in equal proportions which are optically inactive due to the cancellation of rotation of one isomer by the rotation of the other are known as racemic mixtures.
Complete answer:
Isomers are molecules which have the same number of atoms and therefore, they have the same molecular formula. They may have the same molecular formula but they have different structure and arrangement of atoms in a compound. These isomers usually have different physical and chemical properties from each other.
Stereoisomers are types of isomers which have the same molecular formula and same number of atoms but they have different spatial arrangement of atoms that means that they have different three dimensional orientations of atoms in space. Enantiomers are optical isomers which are non-superimposable mirror images. Cis isomers and trans isomers are geometric isomers which differ in terms of position of a group of atoms in a compound.
For the above question,
1,2-dimethyl cyclobutane has one cis and two optically active trans isomers. Optically active trans isomers refer to those trans isomers which cannot be superimposed on their mirror images and are thus chiral and optically active thus, they are capable of optical rotation.




Thus, the correct option is (B) 3, has one cis and two optically active trans forms.
Note:
Stereoisomers which are non superimposable on their mirror images are known as enantiomers and a mixture containing two enantiomers in equal proportions which are optically inactive due to the cancellation of rotation of one isomer by the rotation of the other are known as racemic mixtures.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




