
The total number of ethers possible with the molecular formula ${{C}_{5}}{{H}_{12}}O$ (stereoisomers)
(a) 4
(b) 5
(c) 6
(d) 7
Answer
533.4k+ views
Hint:Stereoisomers are those which has the same molecular formula but different structural formula and by changing the position of the functional group i.e. -O- , you can write many isomers of the given molecular formula. Now write them and then answer the statement accordingly.
Complete step-by-step answer:First of all, let’s discuss what is isomerism. By isomerism , we mean the existence of the different compounds which have the same molecular formula but different properties.
And the Stereoisomerism is that isomerism in which the compounds differ from each other only in the relative arrangement of atoms in space but resemble one another with respect to which atoms are linked to which other atoms.
Now considering the statement as;
Ethers are the compounds which consist of the -C-O-C as the functional group. The number of ethers possible with the molecular formula ${{C}_{5}}{{H}_{12}}O$ is as;
1. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{3}}$
The name of the compound is 1-methoxybutane.
2. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{2}}-C{{H}_{3}}$
The name of the compound is Ethoxy butane.
3.
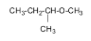
The name of the compound is 2-methoxybutane.
4.
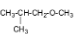
The name of the compound is 3-methoxybutane.
5.
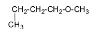
The name of the compound is 4-methoxybutane.
6.
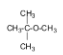
The name of the compound is tert-butyl methyl ether.
So, the total number of ethers possible with the molecular formula ${{C}_{5}}{{H}_{12}}O$ is 6.
Hence, option (c) is correct.
Note:Don’t get confused in the structural and stereoisomerism. Structural isomerism is that type of r isomerism which arises due to the difference in the arrangement of the atoms within the compounds having the same molecular formula . On the other hand, stereoisomerism is that type of isomerism which arises due to the difference in the arrangement of atoms in space.
Complete step-by-step answer:First of all, let’s discuss what is isomerism. By isomerism , we mean the existence of the different compounds which have the same molecular formula but different properties.
And the Stereoisomerism is that isomerism in which the compounds differ from each other only in the relative arrangement of atoms in space but resemble one another with respect to which atoms are linked to which other atoms.
Now considering the statement as;
Ethers are the compounds which consist of the -C-O-C as the functional group. The number of ethers possible with the molecular formula ${{C}_{5}}{{H}_{12}}O$ is as;
1. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{3}}$
The name of the compound is 1-methoxybutane.
2. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{2}}-C{{H}_{3}}$
The name of the compound is Ethoxy butane.
3.

The name of the compound is 2-methoxybutane.
4.
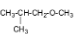
The name of the compound is 3-methoxybutane.
5.
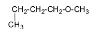
The name of the compound is 4-methoxybutane.
6.

The name of the compound is tert-butyl methyl ether.
So, the total number of ethers possible with the molecular formula ${{C}_{5}}{{H}_{12}}O$ is 6.
Hence, option (c) is correct.
Note:Don’t get confused in the structural and stereoisomerism. Structural isomerism is that type of r isomerism which arises due to the difference in the arrangement of the atoms within the compounds having the same molecular formula . On the other hand, stereoisomerism is that type of isomerism which arises due to the difference in the arrangement of atoms in space.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




