
The total number of cyclic structural, as well as stereoisomers possible for a compound with the molecular formula ${{C}_{5}}{{H}_{10}}$, is ______ .
Answer
581.7k+ views
Hint: Arrange the molecule in as many ring structures as possible. Firstly draw the cyclic structure of ${{C}_{5}}{{H}_{10}}$ and then draw all the cyclic isomers of the molecules by forming a cyclic structure of 3 carbon, 4 carbon and the put the rest no. of carbon as a branch on them. If there were two same branch on a isomers then arrange them in cis-trans form.
Complete answer:
From tour chemistry lessons you have learned about what stereoisomers and which structure is called a cyclic structure.
Isomers are defined as the molecule with the same molecular formula meaning the same no. of atoms but different arrangement of these atoms in space. Stereoisomers are defined as the isomerism in which the molecular formula will be the same but they differ in the three dimensional arrangement of atoms in the space.
Cyclic compounds are those compounds in which one or more atoms are arranged in the manner that one atom in a compound is connected with another to form a ring like structure and the structure formed by a cyclic compound is known as cyclic structure.
In the question we are asked to find all the cyclic structure as well as stereoisomers of ${{C}_{5}}{{H}_{10}}$
Firstly we will draw the cyclic structure of ${{C}_{5}}{{H}_{10}}$ which is,
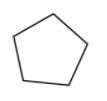
Now we will draw the isomers of this structure without changing the molecular formula, and the cyclic structure of ${{C}_{5}}{{H}_{10}}$ can also be cycloalkanes and not cycloalkenes.
So, the isomers can be 4 carbon ring with a methyl ( $C{{H}_{3}}$) substituent, 3 carbon ring with ethyl substituent (${{C}_{2}}{{H}_{5}}$), 3 carbon ring in which two methyl group is attached with the same carbon, 3 carbon ring in which the two methyl substituent is present in same side of the ring plane (Cis) and a 3 carbon ring in which the two methyl substituent are present on different sides of the ring plane(Trans).
So, the structures of all the isomers is given below,
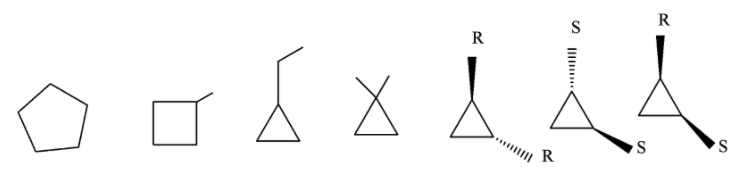
Thus the total no. of cyclic structure as well as stereoisomers isomers is 7.
Note:
The three membered ring itself have total 3 optical isomers (2 cis trans and 1 optical) that is (R,R), (S,S), (R,S), where R and S represents the steroisomers. The IUPAC name of all the structures are Cyclopentane, 1-metrhylcyclobutane, 1-ehylcyclopropane, 1,1-dimethylcyclopropane, 1,2 dimethylcyclopropane (cis-tans).
Complete answer:
From tour chemistry lessons you have learned about what stereoisomers and which structure is called a cyclic structure.
Isomers are defined as the molecule with the same molecular formula meaning the same no. of atoms but different arrangement of these atoms in space. Stereoisomers are defined as the isomerism in which the molecular formula will be the same but they differ in the three dimensional arrangement of atoms in the space.
Cyclic compounds are those compounds in which one or more atoms are arranged in the manner that one atom in a compound is connected with another to form a ring like structure and the structure formed by a cyclic compound is known as cyclic structure.
In the question we are asked to find all the cyclic structure as well as stereoisomers of ${{C}_{5}}{{H}_{10}}$
Firstly we will draw the cyclic structure of ${{C}_{5}}{{H}_{10}}$ which is,

Now we will draw the isomers of this structure without changing the molecular formula, and the cyclic structure of ${{C}_{5}}{{H}_{10}}$ can also be cycloalkanes and not cycloalkenes.
So, the isomers can be 4 carbon ring with a methyl ( $C{{H}_{3}}$) substituent, 3 carbon ring with ethyl substituent (${{C}_{2}}{{H}_{5}}$), 3 carbon ring in which two methyl group is attached with the same carbon, 3 carbon ring in which the two methyl substituent is present in same side of the ring plane (Cis) and a 3 carbon ring in which the two methyl substituent are present on different sides of the ring plane(Trans).
So, the structures of all the isomers is given below,
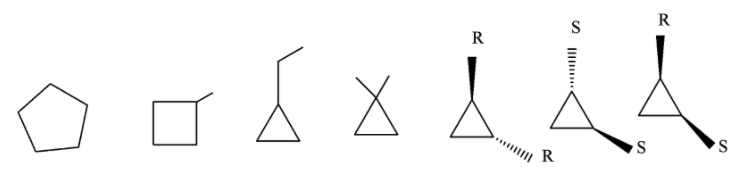
Thus the total no. of cyclic structure as well as stereoisomers isomers is 7.
Note:
The three membered ring itself have total 3 optical isomers (2 cis trans and 1 optical) that is (R,R), (S,S), (R,S), where R and S represents the steroisomers. The IUPAC name of all the structures are Cyclopentane, 1-metrhylcyclobutane, 1-ehylcyclopropane, 1,1-dimethylcyclopropane, 1,2 dimethylcyclopropane (cis-tans).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




