
The sum of the number of lone pairs of electrons on each central atom in the following species is
\[\begin{array}{l}
{{\rm{[TeB}}{{\rm{r}}_{\rm{6}}}]^{2 - }},\;{[{\rm{Br}}{{\rm{F}}_2}]^ + },\;{\rm{SN}}{{\rm{F}}_3},{\rm{ and\;[Xe}}{{\rm{F}}_{\rm{3}}}{]^ - }\\
\left[ {{\rm{Atomic numbers}}:\;{\rm{N}} = 7,\;{\rm{F}} = 9,\;{\rm{S}} = 16,\;{\rm{Br}} = 35,\;{\rm{Te}} = 52,\;{\rm{Xe}} = 54} \right]
\end{array}\]
Answer
571.8k+ views
Hint:As per VSEPR theory this can be solved. We know that VSEPR theory explains the geometry of the molecule. According to this theory, the geometry of the molecule is dependent on the number of bonding and number of non-bonding electron pairs in the central atom.
It is also known to us that the strength of the repulsion between a lone pair and a bond pair of electrons lies in between the repulsion between two lone pairs and between two bond pairs. The order of repulsion between electron pairs as follows: Lone Pair- lone pair > Lone Pair- bond- pair > Bond Pair- bond pair. The electron pairs around the central atom repel each other and move so far apart from each other that there are no greater repulsions between them. This results in the molecule having minimum energy and maximum stability.
Formula used:(a) To find total pairs the formula will be: \[\frac{1}{2}\] (number of valence electrons of central metal atom + number of atoms attached to central metal – overall charge).
(b) Lone pairs (Ip) = Total pairs (T) - Bond pairs (B).
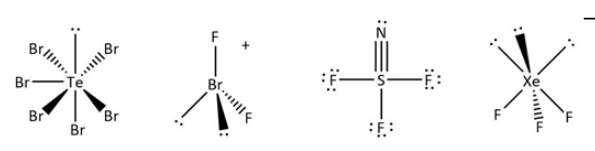
Complete step-by-step answer:The atomic number of \[Te\] atom is \[52\] with electronic configuration \[{\rm{[Kr] 4}}{{\rm{d}}^{{\rm{10}}}}{\rm{\;5}}{{\rm{s}}^{\rm{2}}}{\rm{\;5}}{{\rm{p}}^{\rm{4}}}\], it has six valence electrons hence it will form six bond with bromine. By using this formula (a) and formula (b) we get a number of lone pairs of electrons.
\[{[{\rm{TeB}}{{\rm{r}}_6}]^{2 - }},{\rm{T}} = \frac{1}{2}\left( {6 + 6 + 2} \right) = 7,{\rm{B}} = 6\].
\[{\rm{Te}}\] atom in \[{{\rm{[TeB}}{{\rm{r}}_{\rm{6}}}]^{2 - }}\] has \[1\] lone pair of electrons. Same way we can do it for other molecules: the number of valence electrons for bromine are seven, and two fluorines are attached to it with one positive charge.
\[{[{\rm{Br}}{{\rm{F}}_2}]^ + },{\rm{T}} = \frac{1}{2} = \left( {7 + 2 - 1} \right) = 4,{\rm{B}} = 2\]\[{\rm{Br}}\] atom in\[{[{\rm{Br}}{{\rm{F}}_{\rm{2}}}]^ + }\]has \[2\] lone pairs of electrons.
Xenon has eight electrons in the valence shell. Three fluorines are attached with negative one charge. \[{[{\rm{Xe}}{{\rm{F}}_3}]^ - },T = \frac{1}{2}\left( {8 + 3 + 1} \right) = 6,{\rm{B}} = 3\].\[{\rm{Xe}}\] atom in\[{[{\rm{Xe}}{{\rm{F}}_{\rm{3}}}]^ - }\] has \[3\] lone pairs of electrons.
\[S\]atom in \[SN{F_3}\]has \[0\] lone pair of electrons. Can be concluded from the structure. Because nitrogen and fluorine have different numbers of bond formation with central metal ions.
\[\begin{array}{l}
{\rm{Species}}\;\;\;\;\;\;{[{\rm{TeB}}{{\rm{r}}_6}]^{2 - }}\;\;\;\;{\left[ {{\rm{Br}}{{\rm{F}}_2}} \right]^ + }\,\,\,\,\,\,\,\,\,\,\,{\rm{SN}}{{\rm{F}}_{\rm{3}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\left[ {{\rm{Xe}}{{\rm{F}}_3}} \right]^ - }\\
{\rm{Lone pairs}}\;\;\;\;\;\;\;\;1\;\;\;\;\;\;\;\;\;\;\;\;\;2\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;0\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\,3
\end{array}\]
Hence, the sum of the number of lone pairs of electrons on each central atom in the given species is equal to $6$.
Note:The shape of a molecule with only two atoms is always linear. For molecules with three or more atoms, one of the atoms is called the central atom and other atoms are attached to the central atom. If the central atom is linked to similar atoms and is surrounded by bond pairs of electrons only, the repulsions between them are similar as a result the shape of the molecule is symmetrical and the molecule is said to have regular geometry.
If the central atom is linked to different atoms or is surrounded by bond pair as well as a lone pair of electrons, the repulsion between them is similar. As a result, the shape of the molecule has an irregular or distorted geometry. The exact shape of the molecule depends upon the total number of electron pairs present around the central atom.
It is also known to us that the strength of the repulsion between a lone pair and a bond pair of electrons lies in between the repulsion between two lone pairs and between two bond pairs. The order of repulsion between electron pairs as follows: Lone Pair- lone pair > Lone Pair- bond- pair > Bond Pair- bond pair. The electron pairs around the central atom repel each other and move so far apart from each other that there are no greater repulsions between them. This results in the molecule having minimum energy and maximum stability.
Formula used:(a) To find total pairs the formula will be: \[\frac{1}{2}\] (number of valence electrons of central metal atom + number of atoms attached to central metal – overall charge).
(b) Lone pairs (Ip) = Total pairs (T) - Bond pairs (B).
Complete step-by-step answer:The atomic number of \[Te\] atom is \[52\] with electronic configuration \[{\rm{[Kr] 4}}{{\rm{d}}^{{\rm{10}}}}{\rm{\;5}}{{\rm{s}}^{\rm{2}}}{\rm{\;5}}{{\rm{p}}^{\rm{4}}}\], it has six valence electrons hence it will form six bond with bromine. By using this formula (a) and formula (b) we get a number of lone pairs of electrons.
\[{[{\rm{TeB}}{{\rm{r}}_6}]^{2 - }},{\rm{T}} = \frac{1}{2}\left( {6 + 6 + 2} \right) = 7,{\rm{B}} = 6\].
\[{\rm{Te}}\] atom in \[{{\rm{[TeB}}{{\rm{r}}_{\rm{6}}}]^{2 - }}\] has \[1\] lone pair of electrons. Same way we can do it for other molecules: the number of valence electrons for bromine are seven, and two fluorines are attached to it with one positive charge.
\[{[{\rm{Br}}{{\rm{F}}_2}]^ + },{\rm{T}} = \frac{1}{2} = \left( {7 + 2 - 1} \right) = 4,{\rm{B}} = 2\]\[{\rm{Br}}\] atom in\[{[{\rm{Br}}{{\rm{F}}_{\rm{2}}}]^ + }\]has \[2\] lone pairs of electrons.
Xenon has eight electrons in the valence shell. Three fluorines are attached with negative one charge. \[{[{\rm{Xe}}{{\rm{F}}_3}]^ - },T = \frac{1}{2}\left( {8 + 3 + 1} \right) = 6,{\rm{B}} = 3\].\[{\rm{Xe}}\] atom in\[{[{\rm{Xe}}{{\rm{F}}_{\rm{3}}}]^ - }\] has \[3\] lone pairs of electrons.
\[S\]atom in \[SN{F_3}\]has \[0\] lone pair of electrons. Can be concluded from the structure. Because nitrogen and fluorine have different numbers of bond formation with central metal ions.

\[\begin{array}{l}
{\rm{Species}}\;\;\;\;\;\;{[{\rm{TeB}}{{\rm{r}}_6}]^{2 - }}\;\;\;\;{\left[ {{\rm{Br}}{{\rm{F}}_2}} \right]^ + }\,\,\,\,\,\,\,\,\,\,\,{\rm{SN}}{{\rm{F}}_{\rm{3}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\left[ {{\rm{Xe}}{{\rm{F}}_3}} \right]^ - }\\
{\rm{Lone pairs}}\;\;\;\;\;\;\;\;1\;\;\;\;\;\;\;\;\;\;\;\;\;2\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;0\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\,3
\end{array}\]
Hence, the sum of the number of lone pairs of electrons on each central atom in the given species is equal to $6$.
Note:The shape of a molecule with only two atoms is always linear. For molecules with three or more atoms, one of the atoms is called the central atom and other atoms are attached to the central atom. If the central atom is linked to similar atoms and is surrounded by bond pairs of electrons only, the repulsions between them are similar as a result the shape of the molecule is symmetrical and the molecule is said to have regular geometry.
If the central atom is linked to different atoms or is surrounded by bond pair as well as a lone pair of electrons, the repulsion between them is similar. As a result, the shape of the molecule has an irregular or distorted geometry. The exact shape of the molecule depends upon the total number of electron pairs present around the central atom.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




