
The structures A and B represent
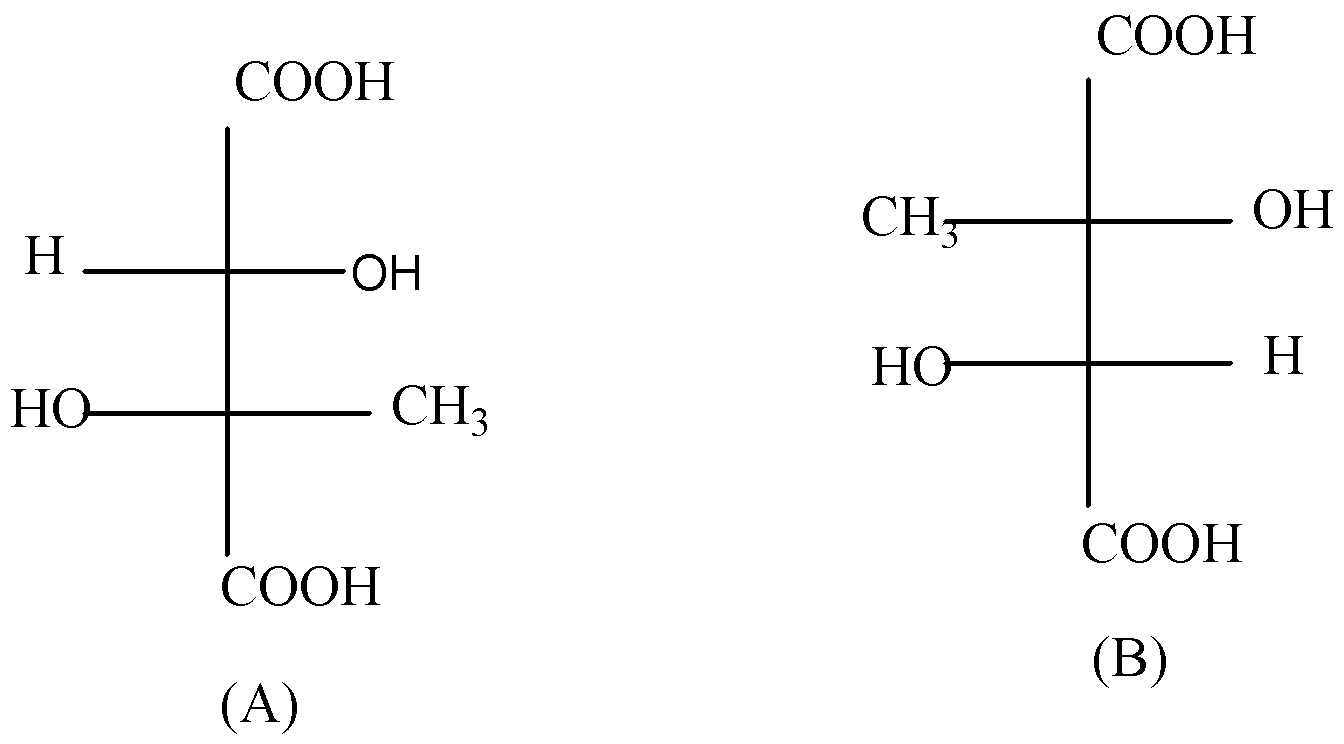
A.Enantiomers
B.Dias termers
C.Homomers
D.Racemic mixture

Answer
582.3k+ views
Hint: The compound A and B are the same Fischer projection because 180° rotations don't change configuration. They are identical and have the same electronic configuration.
Complete step by step answer:
Enantiomers refer to chiral compounds who are mirror images to each other and they rotate polarized light in the opposite direction, but have similar physical and chemical properties.
The compound A and B are not the mirror image to each other and are not superimposable. Hence, structures A and B are not enantiomers.
Diastereomers are stereoisomers of a compound having two or more chiral centers that are not a mirror image of other stereoisomers of the same compound.
Hence, the compound A and B are not Dias termers.
Homomers means two compounds represent in different forms but are exactly the same. The suffix ‘Mers’ comes from a Greek term Meros meaning ‘parts’ or ‘shares. If we rotate one compound by \[180^\circ \] , one will get another compound. So, homomers exist in different forms but are the same compound.
The compound A and B are the same Fischer projection because \[180^\circ \] rotations don’t change configuration. They are identical and have the same electronic configuration. Hence, the compound A and B are homomers.
A racemic mixture containing two enantiomers in equal proportions will have zero optical rotation. Hence, the compound A and B are not racemic mixtures.
Therefore, the correct answer is option (B)
Note: Homomers are the long chain compounds which are made of the same kind of repeating units. Example is polythene which is made up of repeating sections of $C{H_2}$ .
Complete step by step answer:
Enantiomers refer to chiral compounds who are mirror images to each other and they rotate polarized light in the opposite direction, but have similar physical and chemical properties.
The compound A and B are not the mirror image to each other and are not superimposable. Hence, structures A and B are not enantiomers.
Diastereomers are stereoisomers of a compound having two or more chiral centers that are not a mirror image of other stereoisomers of the same compound.
Hence, the compound A and B are not Dias termers.
Homomers means two compounds represent in different forms but are exactly the same. The suffix ‘Mers’ comes from a Greek term Meros meaning ‘parts’ or ‘shares. If we rotate one compound by \[180^\circ \] , one will get another compound. So, homomers exist in different forms but are the same compound.
The compound A and B are the same Fischer projection because \[180^\circ \] rotations don’t change configuration. They are identical and have the same electronic configuration. Hence, the compound A and B are homomers.
A racemic mixture containing two enantiomers in equal proportions will have zero optical rotation. Hence, the compound A and B are not racemic mixtures.
Therefore, the correct answer is option (B)
Note: Homomers are the long chain compounds which are made of the same kind of repeating units. Example is polythene which is made up of repeating sections of $C{H_2}$ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




