
The structure of $Xe{{F}_{6}}$ is experimentally determined to be a distorted octahedron. It's structure according to VSEPR theory is:
A. Octahedron
B. Original bipyramid
C. Pentagonal bipyramid
D. Tetragonal bipyramid
Answer
591.3k+ views
Hint: In $Xe{{F}_{6}}$, think about the electronic configuration, the number of valence electrons and the lone pairs present. Also consider the number of electrons used up in bonding with fluorine.
Complete answer:
First, let us look at the electronic configuration and the valence electrons of xenon. We know that it is a p-block element and is present in group 18. Thus, it will have 8 valence electrons. The electronic configuration is $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$. Thus, shell number 5 has 8 electrons. Out of these, 6 will be used up in binding with fluorine atoms. The remaining 2 will be a lone pair.
Now, we will calculate the geometry of the structure by calculating all the pairs of electrons involved.
Total pairs = No. of bond pairs + No. of lone pairs
Total pairs on $Xe$ = 6 bond pairs with $F$ + 1 lone pair
Total pairs on $Xe$ = 7 pairs
Thus, the geometry of the complex will be pentagonal bipyramidal. The structure of this molecule is as follows:
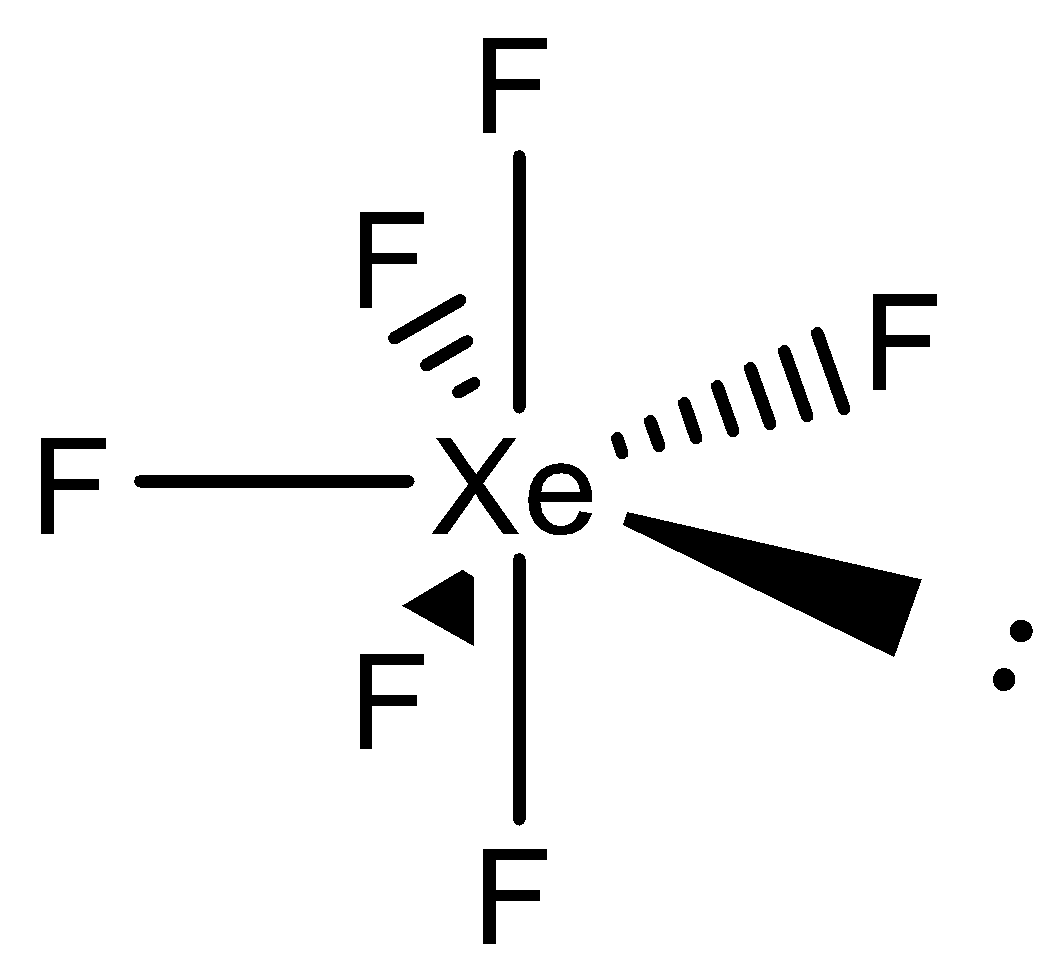
So, the structure of $Xe{{F}_{6}}$ is the Pentagonal Bipyramid.
Therefore, ‘Option C’ is the correct answer.
Additional Information:
VSEPR or Valence Shell Electron Pair Repulsion Theory is a model used to predict the geometry of molecules based on minimizing the electrostatic repulsion of a molecule’s valence electrons around a central atom.
The purpose of Valence Shell Electron Pair Repulsion Theory is used in chemistry to predict the three-dimensional shapes of molecules.
Note:
Do not get confused since the electronic configuration of $Xe$ shows 10 electrons placed in the $4d$ orbital. We only need to consider the electrons in the outermost shell while calculating the number of bond pairs and lone pairs since they are the only ones that contribute to the geometry of the molecule.
Complete answer:
First, let us look at the electronic configuration and the valence electrons of xenon. We know that it is a p-block element and is present in group 18. Thus, it will have 8 valence electrons. The electronic configuration is $[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$. Thus, shell number 5 has 8 electrons. Out of these, 6 will be used up in binding with fluorine atoms. The remaining 2 will be a lone pair.
Now, we will calculate the geometry of the structure by calculating all the pairs of electrons involved.
Total pairs = No. of bond pairs + No. of lone pairs
Total pairs on $Xe$ = 6 bond pairs with $F$ + 1 lone pair
Total pairs on $Xe$ = 7 pairs
Thus, the geometry of the complex will be pentagonal bipyramidal. The structure of this molecule is as follows:

So, the structure of $Xe{{F}_{6}}$ is the Pentagonal Bipyramid.
Therefore, ‘Option C’ is the correct answer.
Additional Information:
VSEPR or Valence Shell Electron Pair Repulsion Theory is a model used to predict the geometry of molecules based on minimizing the electrostatic repulsion of a molecule’s valence electrons around a central atom.
The purpose of Valence Shell Electron Pair Repulsion Theory is used in chemistry to predict the three-dimensional shapes of molecules.
Note:
Do not get confused since the electronic configuration of $Xe$ shows 10 electrons placed in the $4d$ orbital. We only need to consider the electrons in the outermost shell while calculating the number of bond pairs and lone pairs since they are the only ones that contribute to the geometry of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




