
The structure of $Xe{{F}_{2}}$ involves hybridization of the type:
[A] $s{{p}^{3}}$
[B] $ds{{p}^{2}}$
[C] $s{{p}^{3}}d$
[D] $s{{p}^{3}}{{d}^{2}}$
Answer
589.8k+ views
Hint: To find the hybridisation of any complex, we need to determine the coordination number of the complex. If we know the coordination number, we can easily find out the hybridization and the geometry of the complex according to the valence bond theory.
Complete step by step answer:
In the valence bond theory, as we know atomic orbitals overlap with other atomic orbitals to form a molecule and thus creating new hybrid orbitals. This is known as the phenomenon of hybridisation.
The compound given to us is $Xe{{F}_{2}}$. To find its hybridisation, firstly let us write down its electronic configuration of the central metal atom that is xenon in this case.
Tellurium belongs to group 18 and its atomic number is 54. So, we can write its electronic configuration as- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$.
We can see that there are 8 electrons in the valence shell. And from each fluorine atom we will have an electronic contribution of 1. So, from the two fluorine atoms we have a total contribution of 2.
So, the total number of electrons is 8 + 2 = 10. Or we can say that the number of electron pairs is 5.
Therefore, the coordination number is 5.
For coordination number 5, the corresponding hybridisation is $s{{p}^{3}}d$ and the shape is trigonal bipyramidal.
So, we can draw its structure as-
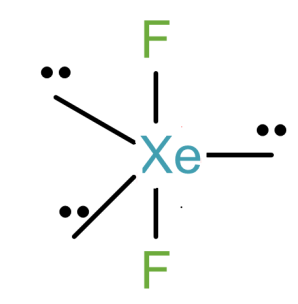
From this we can understand that the hybridisation is $s{{p}^{3}}d$
So, the correct answer is “Option C”.
Note: We can use the VSEPR theory which is the valence shell electron pair repulsion theory to correlate the hybridisation with the shape as well as the geometry of a molecule. Molecules settle in a shape where the electronic repulsion is the minimum and thus creates as lower energy shape as possible. Hybridization affects the bonds and the types of the bonds the molecule makes and thus is correlated to the shape of the molecule.
Complete step by step answer:
In the valence bond theory, as we know atomic orbitals overlap with other atomic orbitals to form a molecule and thus creating new hybrid orbitals. This is known as the phenomenon of hybridisation.
The compound given to us is $Xe{{F}_{2}}$. To find its hybridisation, firstly let us write down its electronic configuration of the central metal atom that is xenon in this case.
Tellurium belongs to group 18 and its atomic number is 54. So, we can write its electronic configuration as- $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}$.
We can see that there are 8 electrons in the valence shell. And from each fluorine atom we will have an electronic contribution of 1. So, from the two fluorine atoms we have a total contribution of 2.
So, the total number of electrons is 8 + 2 = 10. Or we can say that the number of electron pairs is 5.
Therefore, the coordination number is 5.
For coordination number 5, the corresponding hybridisation is $s{{p}^{3}}d$ and the shape is trigonal bipyramidal.
So, we can draw its structure as-

From this we can understand that the hybridisation is $s{{p}^{3}}d$
So, the correct answer is “Option C”.
Note: We can use the VSEPR theory which is the valence shell electron pair repulsion theory to correlate the hybridisation with the shape as well as the geometry of a molecule. Molecules settle in a shape where the electronic repulsion is the minimum and thus creates as lower energy shape as possible. Hybridization affects the bonds and the types of the bonds the molecule makes and thus is correlated to the shape of the molecule.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




