
The structure of spiro $\left[ {3,3} \right]$ heptane is:
A.
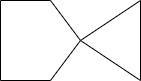
B.
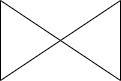
C.
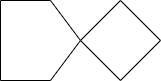
D.
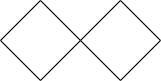




Answer
577.8k+ views
Hint: Spiro compounds are the bi- or polycyclic organic compounds with rings connected through just one atom. The rings can be different in nature or identical. The connecting atom is also called the spiro atom or spiro carbon, most often a quaternary carbon.
Complete step by step solution:
There are two types of spiro compounds. They are mono and poly spiro compounds. Mono spiro compounds are the compounds having two rings. Poly spiro compounds are the compounds having three or more spiro compounds.
The nomenclature was proposed by Adolf von Baeyer. Spiro compounds’ names have three parts-spiro prefix, square bracket and hydrocarbon name. All spiro compounds have the prefix spiro followed by square brackets containing the number of atoms in the smaller ring and the number of atoms in the larger ring excluding the spiro atom itself, the numbers being separated by a dot. Bridge-head carbons are where the rings meet. It is different from bicyclic compounds. Spiro is put as a prefix and insert the two bridge lengths and place the suffix with respect to the number of carbons. In the square brackets, the number of carbon atoms linked to the spiro atom in each ring is indicated in ascending order.
Since $\left[ {3,3} \right]$ denotes the number of carbons linked to spiro atom, there are three carbons
near to the spiro carbon,
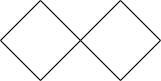
which will be the structure. So except the spiro carbon atom, there are six carbons, thus the name heptane.
Hence the correct option is D.
Note: We can obtain spiro compounds using intramolecular cyclization, intra/intermolecular condensation. Isolated rings do not have common atoms. Spiro rings have one common atom and fused rings have one common bond. While bridged rings have two common atoms.
Complete step by step solution:
There are two types of spiro compounds. They are mono and poly spiro compounds. Mono spiro compounds are the compounds having two rings. Poly spiro compounds are the compounds having three or more spiro compounds.
The nomenclature was proposed by Adolf von Baeyer. Spiro compounds’ names have three parts-spiro prefix, square bracket and hydrocarbon name. All spiro compounds have the prefix spiro followed by square brackets containing the number of atoms in the smaller ring and the number of atoms in the larger ring excluding the spiro atom itself, the numbers being separated by a dot. Bridge-head carbons are where the rings meet. It is different from bicyclic compounds. Spiro is put as a prefix and insert the two bridge lengths and place the suffix with respect to the number of carbons. In the square brackets, the number of carbon atoms linked to the spiro atom in each ring is indicated in ascending order.
Since $\left[ {3,3} \right]$ denotes the number of carbons linked to spiro atom, there are three carbons
near to the spiro carbon,

which will be the structure. So except the spiro carbon atom, there are six carbons, thus the name heptane.
Hence the correct option is D.
Note: We can obtain spiro compounds using intramolecular cyclization, intra/intermolecular condensation. Isolated rings do not have common atoms. Spiro rings have one common atom and fused rings have one common bond. While bridged rings have two common atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




