
The structure of ${\text{I}}{{\text{F}}_{\text{5}}}$ can be best demonstrated as:
(A)-
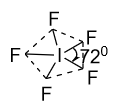
(B)-
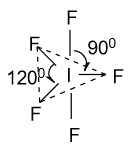
(C)-
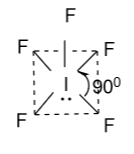
(D)- None of these



Answer
554.1k+ views
Hint: For the estimation of exact structure of any compound we have to know about the atomic number electronic configuration of all the atoms present in that compound, so that we will assure the bonding between them and will predict the geometry and structure.
Complete answer:
For the structural demonstration of ${\text{I}}{{\text{F}}_{\text{5}}}$, we have to consider following points:
-In ${\text{I}}{{\text{F}}_{\text{5}}}$ iodine is the central atom as it is electropositive or less electronegative as compare to the fluorine atom.
-Atomic number of fluorine is 9 and its electronic configuration is written as ${\text{1}}{{\text{s}}^{\text{2}}}{\text{,2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{5}}}$, from this it is clear that in the outermost shell of fluorine seven valence electrons are present.
-Atomic number of iodine is 53 and its electronic configuration is written as $\left[ {{\text{Kr}}} \right]{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{s}}^{\text{2}}}{\text{5}}{{\text{p}}^{\text{5}}}$, from this it is clear that in the outermost shell of iodine also seven valence electrons are present.
-Out of these seven valence electrons of iodine five makes bonds with five fluorine atoms in the excited state and remaining two electrons are present in the form of lone pair in the following manner:
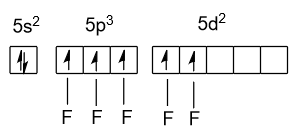
-Five bond pairs and one electron lone pair of iodine produces ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ which demonstrate the structure of square pyramidal.
Hence, option (C) is correct.
Note:
Here some of you may think that the term geometry and structure convey the same meaning but it is not true. The geometry of any compound shows the diagram of the compound when all bond pairs are present, but if some deflection arises in the compound due to the presence of lone pair or any other species then the obtained diagram will show the structure of that compound.
Complete answer:
For the structural demonstration of ${\text{I}}{{\text{F}}_{\text{5}}}$, we have to consider following points:
-In ${\text{I}}{{\text{F}}_{\text{5}}}$ iodine is the central atom as it is electropositive or less electronegative as compare to the fluorine atom.
-Atomic number of fluorine is 9 and its electronic configuration is written as ${\text{1}}{{\text{s}}^{\text{2}}}{\text{,2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{5}}}$, from this it is clear that in the outermost shell of fluorine seven valence electrons are present.
-Atomic number of iodine is 53 and its electronic configuration is written as $\left[ {{\text{Kr}}} \right]{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{s}}^{\text{2}}}{\text{5}}{{\text{p}}^{\text{5}}}$, from this it is clear that in the outermost shell of iodine also seven valence electrons are present.
-Out of these seven valence electrons of iodine five makes bonds with five fluorine atoms in the excited state and remaining two electrons are present in the form of lone pair in the following manner:

-Five bond pairs and one electron lone pair of iodine produces ${\text{s}}{{\text{p}}^{\text{3}}}{{\text{d}}^{\text{2}}}$ which demonstrate the structure of square pyramidal.
Hence, option (C) is correct.
Note:
Here some of you may think that the term geometry and structure convey the same meaning but it is not true. The geometry of any compound shows the diagram of the compound when all bond pairs are present, but if some deflection arises in the compound due to the presence of lone pair or any other species then the obtained diagram will show the structure of that compound.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




