
The structural formula of mesityl oxide is:
Answer
561k+ views
Hint The mesityl oxide is the compound that belongs to the $ \alpha ,\beta $ -unsaturated ketone means the carbon atom having the ketone group is the alpha-carbon and the beta-carbon will have the double bond. The molecular form of the mesityl oxide is $ {{C}_{6}}{{H}_{10}}O $ .
Complete step by step answer:
The mesityl oxide is the compound that belongs to the $ \alpha ,\beta $ -unsaturated ketone means the carbon atom having the ketone group is the alpha-carbon and the beta-carbon will have the double bond. The molecular formula of mesityl oxide is $ {{C}_{6}}{{H}_{10}}O $ and the IUPAC name of mesityl oxide is 4-methylpent-3-en-2-one. According to the name, the structural formula of the mesityl oxide will be:
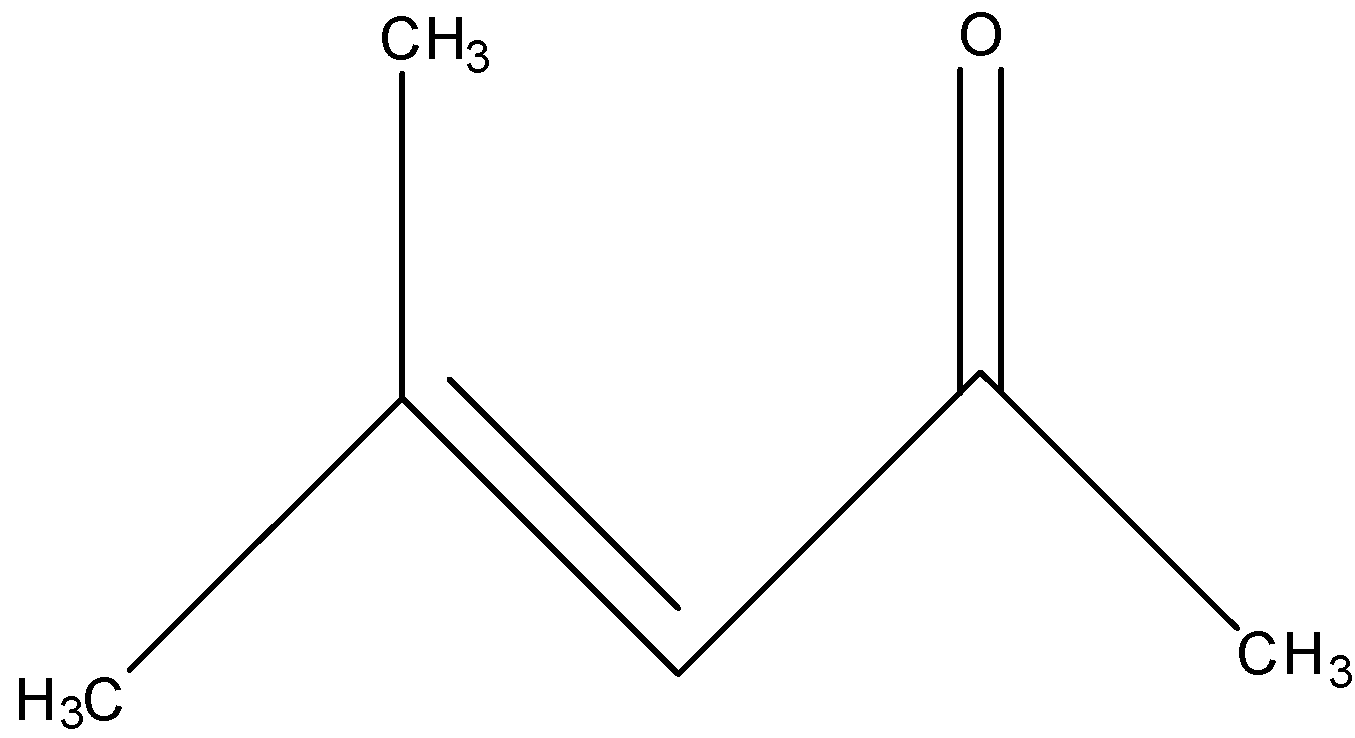
The other names of the mesityl oxide are isobutenyl methyl ketone, methyl isobutenyl ketone, and isopropylidene acetone.
The molecular mass of the mesityl oxide is 98.145 g/mol. The texture of the mesityl oxide is oily and its color ranges from colorless to light yellow, and it is a liquid.
It has a honey-like or peppermint like odor.
The density of the mesityl oxide is 0.858 $ g/c{{m}^{3}} $ .
The melting point of the mesityl oxide is 220 K or $ -{{53}^{\circ }}C $ and its boiling point are 402.6 K.
It is not soluble in water or its solubility is only 3% but in other solvents like organic solvents, it is soluble. The refractive index of mesityl oxide is 1.442. Its vapor pressure at 293 K is 9 mmHg. It is a flammable compound.
Note: It is mainly prepared by the aldol condensation of the acetone to give diacetone alcohol, which dehydrated to give mesityl oxide and the main use of mesityl oxide is as a solvent i.e., it is used as solvents in many reactions.
Complete step by step answer:
The mesityl oxide is the compound that belongs to the $ \alpha ,\beta $ -unsaturated ketone means the carbon atom having the ketone group is the alpha-carbon and the beta-carbon will have the double bond. The molecular formula of mesityl oxide is $ {{C}_{6}}{{H}_{10}}O $ and the IUPAC name of mesityl oxide is 4-methylpent-3-en-2-one. According to the name, the structural formula of the mesityl oxide will be:

The other names of the mesityl oxide are isobutenyl methyl ketone, methyl isobutenyl ketone, and isopropylidene acetone.
The molecular mass of the mesityl oxide is 98.145 g/mol. The texture of the mesityl oxide is oily and its color ranges from colorless to light yellow, and it is a liquid.
It has a honey-like or peppermint like odor.
The density of the mesityl oxide is 0.858 $ g/c{{m}^{3}} $ .
The melting point of the mesityl oxide is 220 K or $ -{{53}^{\circ }}C $ and its boiling point are 402.6 K.
It is not soluble in water or its solubility is only 3% but in other solvents like organic solvents, it is soluble. The refractive index of mesityl oxide is 1.442. Its vapor pressure at 293 K is 9 mmHg. It is a flammable compound.
Note: It is mainly prepared by the aldol condensation of the acetone to give diacetone alcohol, which dehydrated to give mesityl oxide and the main use of mesityl oxide is as a solvent i.e., it is used as solvents in many reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




