
The standard molar enthalpies of formation of cyclohexane (l) and benzene (l) at ${ 25 }^{ \circ }{ C }$ are ${ -156 }$ and ${ 49kJmol }^{ -1 }$ respectively. The standard enthalpy of hydrogenation of cyclohexene (l) at ${ 25 }^{ \circ }{ C }$ is ${ -119kJmol }^{ -1 }$. Use this data to estimate the magnitude of the resonance energy of benzene.
Answer
579.3k+ views
Hint: To answer this, you must know what enthalpy of formation and heat of hydrogenation is.
Remember that resonance energy is given by ${ \Delta H }_{ obs }{ -\Delta H }_{ cal }$. To find this, use the formula-
$\Delta {{H}_{cal}}=\Delta {{H}_{f}}\text{ }of\text{ }cyclohexane-\Delta {{H}_{f}}\text{ }of\text{ }benzene+3\times \Delta {{H}_{f}}\text{ }of\text{ }{{H}_{2}}$ and remember that $\Delta {{H}_{obs}}$ is the observed value which you can find out from the standard enthalpy of hydrogenation of cyclohexene.
Complete Solution :
Firstly, let us discuss what hydrogenation and enthalpy of formation is.
At these standard conditions we can define the standard enthalpy of formation as the change of enthalpy of during the formation of 1 mole of the substance where all the substances are in their standard states.
Hydrogenation of alkenes simply means addition of hydrogen atoms across the double bonds. The product thus formed has a carbon-carbon single covalent bond and is known as an alkane. Addition of hydrogen atoms across the double bond will cause loss of a bond.
Now, we can see that in the question, it is given to us that-
Standard molar enthalpy of formation of cyclohexane = ${ -156kJmol }^{ -1 }$
Standard molar enthalpy of formation of benzene = ${ 49kJmol }^{ -1 }$
Standard enthalpy of hydrogenation of cyclohexene = ${ -119kJmol }^{ -1 }$
Now, if we write the reaction for these processes according to the definition, it will be-
${ 6C+6H }_{ 2 }{ \rightarrow C }_{ 6 }{ H }_{ 12 }{ ;\Delta H=-156kJmol }^{ -1 }$
${ 6C+3H }_{ 2 }{ \rightarrow C }_{ 6 }{ H }_{ 6 }{ ;\Delta H=49kJmol }^{ -1 }$
${ { C }_{ 6 }{ H }_{ 10 }+H }_{ 2 }{ \rightarrow C }_{ 6 }{ H }_{ 12 }{ ;\Delta H=-119kJmol }^{ -1 }$
We have to find out the resonance energy of benzene i.e. we have to calculate -
${{C}_{6}}{{H}_{6}}(l)+3{{H}_{2}}\to {{C}_{6}}{{H}_{12}};\text{ }\Delta H=?$
- Now, ${ \Delta H }$ can be calculated by-
$\Delta {{H}_{obs}}=\Delta {{H}_{f}}\text{ }of\text{ }cyclohexane-\Delta {{H}_{f}}\text{ }of\text{ }benzene+3\times \Delta {{H}_{f}}\text{ }of\text{ }{{H}_{2}}$
${ \Delta H }_{ cal }$ = $[-156-(+49)+\dfrac{3}{0}]$
${ \Delta H }_{ cal }$ = ${ -205kJmol }^{ -1 }$
Since the double bond of cyclohexane is hydrogenated and its standard enthalpy of hydrogenation = ${ -119 kJmol }^{ -1 }$
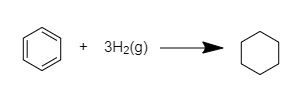
In this, three C=C bonds are present in benzene and by hydrogenation, they can change into benzene.
Thus, the ${ \Delta H }$ of this heat of hydrogenation = ${ 3\times (-119kJmol^{ -1 }) }$= ${ -357kJmol }^{ -1 }$
As we know resonance energy = ${ \Delta H }_{ obs }{ -\Delta H }_{ cal }$
= ${ [-357- (-205)] }$ = ${ -152kJmol }^{ -1 }$
Hence, the resonance energy of benzene = ${ -152kJmol }^{ -1 }$
Additional Information:
- The heat of hydrogenation is the amount of heat released during the hydrogenation of an alkene. It depends on the stability of the carbon.
- The less stable i.e. less substituted carbon will have a higher heat of hydrogenation whereas the more stable i.e. more substituted carbon will have less heat of hydrogenation.
Note: The possibility to make a mistake is that when you calculate ${ \Delta H }_{ obs }$ , three hydrogens are required for hydrogenation, so you have to multiply the standard enthalpy of hydration with three to calculate this heat of hydrogenation.
Remember that resonance energy is given by ${ \Delta H }_{ obs }{ -\Delta H }_{ cal }$. To find this, use the formula-
$\Delta {{H}_{cal}}=\Delta {{H}_{f}}\text{ }of\text{ }cyclohexane-\Delta {{H}_{f}}\text{ }of\text{ }benzene+3\times \Delta {{H}_{f}}\text{ }of\text{ }{{H}_{2}}$ and remember that $\Delta {{H}_{obs}}$ is the observed value which you can find out from the standard enthalpy of hydrogenation of cyclohexene.
Complete Solution :
Firstly, let us discuss what hydrogenation and enthalpy of formation is.
At these standard conditions we can define the standard enthalpy of formation as the change of enthalpy of during the formation of 1 mole of the substance where all the substances are in their standard states.
Hydrogenation of alkenes simply means addition of hydrogen atoms across the double bonds. The product thus formed has a carbon-carbon single covalent bond and is known as an alkane. Addition of hydrogen atoms across the double bond will cause loss of a bond.
Now, we can see that in the question, it is given to us that-
Standard molar enthalpy of formation of cyclohexane = ${ -156kJmol }^{ -1 }$
Standard molar enthalpy of formation of benzene = ${ 49kJmol }^{ -1 }$
Standard enthalpy of hydrogenation of cyclohexene = ${ -119kJmol }^{ -1 }$
Now, if we write the reaction for these processes according to the definition, it will be-
${ 6C+6H }_{ 2 }{ \rightarrow C }_{ 6 }{ H }_{ 12 }{ ;\Delta H=-156kJmol }^{ -1 }$
${ 6C+3H }_{ 2 }{ \rightarrow C }_{ 6 }{ H }_{ 6 }{ ;\Delta H=49kJmol }^{ -1 }$
${ { C }_{ 6 }{ H }_{ 10 }+H }_{ 2 }{ \rightarrow C }_{ 6 }{ H }_{ 12 }{ ;\Delta H=-119kJmol }^{ -1 }$
We have to find out the resonance energy of benzene i.e. we have to calculate -
${{C}_{6}}{{H}_{6}}(l)+3{{H}_{2}}\to {{C}_{6}}{{H}_{12}};\text{ }\Delta H=?$
- Now, ${ \Delta H }$ can be calculated by-
$\Delta {{H}_{obs}}=\Delta {{H}_{f}}\text{ }of\text{ }cyclohexane-\Delta {{H}_{f}}\text{ }of\text{ }benzene+3\times \Delta {{H}_{f}}\text{ }of\text{ }{{H}_{2}}$
${ \Delta H }_{ cal }$ = $[-156-(+49)+\dfrac{3}{0}]$
${ \Delta H }_{ cal }$ = ${ -205kJmol }^{ -1 }$
Since the double bond of cyclohexane is hydrogenated and its standard enthalpy of hydrogenation = ${ -119 kJmol }^{ -1 }$
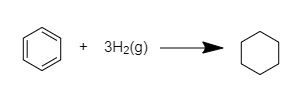
In this, three C=C bonds are present in benzene and by hydrogenation, they can change into benzene.
Thus, the ${ \Delta H }$ of this heat of hydrogenation = ${ 3\times (-119kJmol^{ -1 }) }$= ${ -357kJmol }^{ -1 }$
As we know resonance energy = ${ \Delta H }_{ obs }{ -\Delta H }_{ cal }$
= ${ [-357- (-205)] }$ = ${ -152kJmol }^{ -1 }$
Hence, the resonance energy of benzene = ${ -152kJmol }^{ -1 }$
Additional Information:
- The heat of hydrogenation is the amount of heat released during the hydrogenation of an alkene. It depends on the stability of the carbon.
- The less stable i.e. less substituted carbon will have a higher heat of hydrogenation whereas the more stable i.e. more substituted carbon will have less heat of hydrogenation.
Note: The possibility to make a mistake is that when you calculate ${ \Delta H }_{ obs }$ , three hydrogens are required for hydrogenation, so you have to multiply the standard enthalpy of hydration with three to calculate this heat of hydrogenation.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




