
The stability of benzene diazonium salts is because of:
(A) Inductive Effect.
(B) Resonance.
(C) Hyperconjugation.
(D) Mesomeric Effect.
Answer
534.6k+ views
Hint: We know that In order to answer the question, first we have to check whether the given salt is more stable than others. Then after we will discuss the most stable diazonium salts. This is not found in aliphatic diazonium salts.
Complete answer:
Aromatic diazonium salts such as Benzene Diazonium Halide are more stable than aliphatic diazonium salts due to dispersion of positive charge over the benzene ring caused by resonance. Dispersion of positive charge over the benzene ring caused by resonance. Diazonium salts are the organic compounds consisting of triple bonds in between Nitrogen atoms as well as having either an alkyl or aryl (benzene ring) on the other side.
The diazonium salts are the moderate stage between the azo colors (or mixes are known to be mainstream shading specialists). Diazonium salt containing aryl bunch straightforwardly connected to the nitrogen iota is generally steady because of reverberation adjustment between the benzene cores and N-molecule. And the Benzene Diazonium Halide has a N,N triple bond and containing an aryl group directly linked to the Nitrogen atom makes it more stable than other given Diazonium Salts.
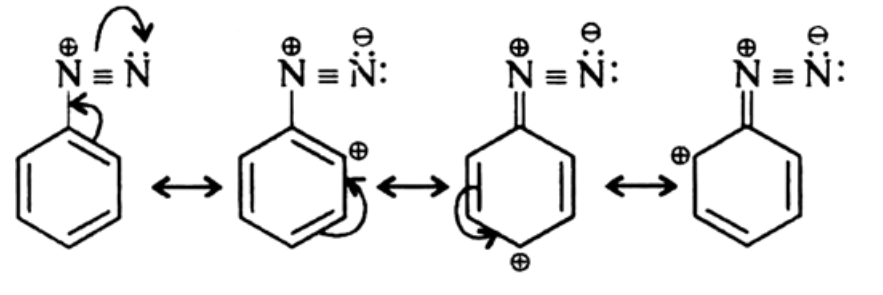
Due to resonance there is a dispersal of positive charge on the benzene ring. This resonance accounts for the stability of the diazonium ion. Hence, diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Therefore, the correct answer is option B.
Note:
Remember that one of the most widely recognized strategies for arrangement of diazonium salt is by the response of nitrous corrosive with fragrant amines. The response of aniline (sweet-smelling amine) with nitrous corrosive outcomes in the arrangement of the diazonium salt. This salt is the benzene diazonium chloride. Nitrous corrosive is a profoundly poisonous gas.
Complete answer:
Aromatic diazonium salts such as Benzene Diazonium Halide are more stable than aliphatic diazonium salts due to dispersion of positive charge over the benzene ring caused by resonance. Dispersion of positive charge over the benzene ring caused by resonance. Diazonium salts are the organic compounds consisting of triple bonds in between Nitrogen atoms as well as having either an alkyl or aryl (benzene ring) on the other side.
The diazonium salts are the moderate stage between the azo colors (or mixes are known to be mainstream shading specialists). Diazonium salt containing aryl bunch straightforwardly connected to the nitrogen iota is generally steady because of reverberation adjustment between the benzene cores and N-molecule. And the Benzene Diazonium Halide has a N,N triple bond and containing an aryl group directly linked to the Nitrogen atom makes it more stable than other given Diazonium Salts.

Due to resonance there is a dispersal of positive charge on the benzene ring. This resonance accounts for the stability of the diazonium ion. Hence, diazonium salts of aromatic amines are more stable than those of aliphatic amines.
Therefore, the correct answer is option B.
Note:
Remember that one of the most widely recognized strategies for arrangement of diazonium salt is by the response of nitrous corrosive with fragrant amines. The response of aniline (sweet-smelling amine) with nitrous corrosive outcomes in the arrangement of the diazonium salt. This salt is the benzene diazonium chloride. Nitrous corrosive is a profoundly poisonous gas.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




