
The species having pyramidal shape is:
(A) $S{{O}_{3}}$
(B) $Br{{F}_{3}}$
(C) $Si{{O}_{3}}^{2-}$
(D) $OS{{F}_{2}}$
Answer
581.4k+ views
Hint: Lewis's concept is unable to explain the shapes of molecules. The valence shell electron pair repulsion theory provides a simple procedure to identify the shapes of covalent molecules. This theory is based on the repulsive interactions of electron pairs in the valence shell of the central atom.
Complete answer:
According to VSEPR theory, the valence electron pairs in a molecule will arrange themselves around the central atoms of the molecule and so that they are as far apart as they can be.
Postulates of VSEPR theory:
(i) The unpaired electrons in the valence shell of the central atom from a bond with the unpaired electron of surrounding atoms, whereas paired electrons remain as lone pairs in the valence shell of the central atom.
(ii) The electrons in the orbital of the valence shell of the central atom should be arranged in a space such a way that hybrid orbitals lie as far away from one another as to give maximum stability to the molecule.
(iii) Lone pair and bond pair repel each other.
(iv) the number of bond pairs, as well as lone pairs surrounded by the central atom, decides the geometry and shape of the molecule.
(v) Electron pairs around the central atom should adjust themselves to reduce the minimum repulsive forces around the central atom.
(vi) there are three types of repulsions possible, lp-lp, lp-bp, bp-bp and the magnitude of repulsive forces are different as follows: $lp-lp>lp-bp>bp-bp$
Given, $S{{O}_{3}}$ a molecule has hybridization is $s{{p}^{2}}$ and the shape of the molecule is trigonal planar,
$Br{{F}_{3}}$ the molecule has hybridization is $s{{p}^{3}}d$ and the shape of the molecule is distorted T-shape, because of the repulsion between 3 bond pairs and two lone pairs.
$Si{{O}_{3}}^{2-}$ a molecule, the silicon hybridization is $s{{p}^{3}}$ and four oxygen atoms arranged tetrahedrally around silicon atom.
$OS{{F}_{2}}$ has $s{{p}^{3}}$ hybridization, but the structure is distorted and the tetrahedral becomes a pyramidal shape.
According to the VSEPR theory, the repulsion between the lone pair and three bond pairs, the structure changed to the pyramid shape.
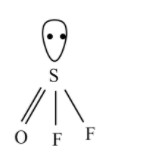
Hence, the correct answer is option D.
Note:
Determining the interactions and reactions of the molecules with other molecules, its shape plays an important role and influences the boiling point and melting point of molecules. The property of molecules depends on a particular shape and forms the shape which defines many properties.
Complete answer:
According to VSEPR theory, the valence electron pairs in a molecule will arrange themselves around the central atoms of the molecule and so that they are as far apart as they can be.
Postulates of VSEPR theory:
(i) The unpaired electrons in the valence shell of the central atom from a bond with the unpaired electron of surrounding atoms, whereas paired electrons remain as lone pairs in the valence shell of the central atom.
(ii) The electrons in the orbital of the valence shell of the central atom should be arranged in a space such a way that hybrid orbitals lie as far away from one another as to give maximum stability to the molecule.
(iii) Lone pair and bond pair repel each other.
(iv) the number of bond pairs, as well as lone pairs surrounded by the central atom, decides the geometry and shape of the molecule.
(v) Electron pairs around the central atom should adjust themselves to reduce the minimum repulsive forces around the central atom.
(vi) there are three types of repulsions possible, lp-lp, lp-bp, bp-bp and the magnitude of repulsive forces are different as follows: $lp-lp>lp-bp>bp-bp$
Given, $S{{O}_{3}}$ a molecule has hybridization is $s{{p}^{2}}$ and the shape of the molecule is trigonal planar,
$Br{{F}_{3}}$ the molecule has hybridization is $s{{p}^{3}}d$ and the shape of the molecule is distorted T-shape, because of the repulsion between 3 bond pairs and two lone pairs.
$Si{{O}_{3}}^{2-}$ a molecule, the silicon hybridization is $s{{p}^{3}}$ and four oxygen atoms arranged tetrahedrally around silicon atom.
$OS{{F}_{2}}$ has $s{{p}^{3}}$ hybridization, but the structure is distorted and the tetrahedral becomes a pyramidal shape.
According to the VSEPR theory, the repulsion between the lone pair and three bond pairs, the structure changed to the pyramid shape.

Hence, the correct answer is option D.
Note:
Determining the interactions and reactions of the molecules with other molecules, its shape plays an important role and influences the boiling point and melting point of molecules. The property of molecules depends on a particular shape and forms the shape which defines many properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




