
The silicate anion in the mineral kinoite is a chain of three \[Si{O_4}^{4 - }\] tetrahedral that share corners with adjacent tetrahedral. The charge of the silicate anion is:
A.$ - 4$
B.$ - 8$
C.$ - 6$
D.$ - 2$
Answer
516.3k+ views
Hint: We have to know that the building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom. These are arranged such that planes drawn through the oxygen atoms form a tetrahedron.
Complete answer:
We have to know that the kinoite mineral has a molecular formula \[C{a_2}C{u_2}S{i_3}{O_8}{(OH)_4}\] it is a bluish colored copper silicate mineral. The crystal structure of this mineral is monoclinic crystal. It is lustrous in appearance and also somewhat transparent.
Now solving the question given it is given that kyanite mineral is a chain of three \[Si{O_4}^{4 - }\] tetrahedral that share corners with adjacent tetrahedral, thus first we need to draw what is asked that makes it easy to answer this question:
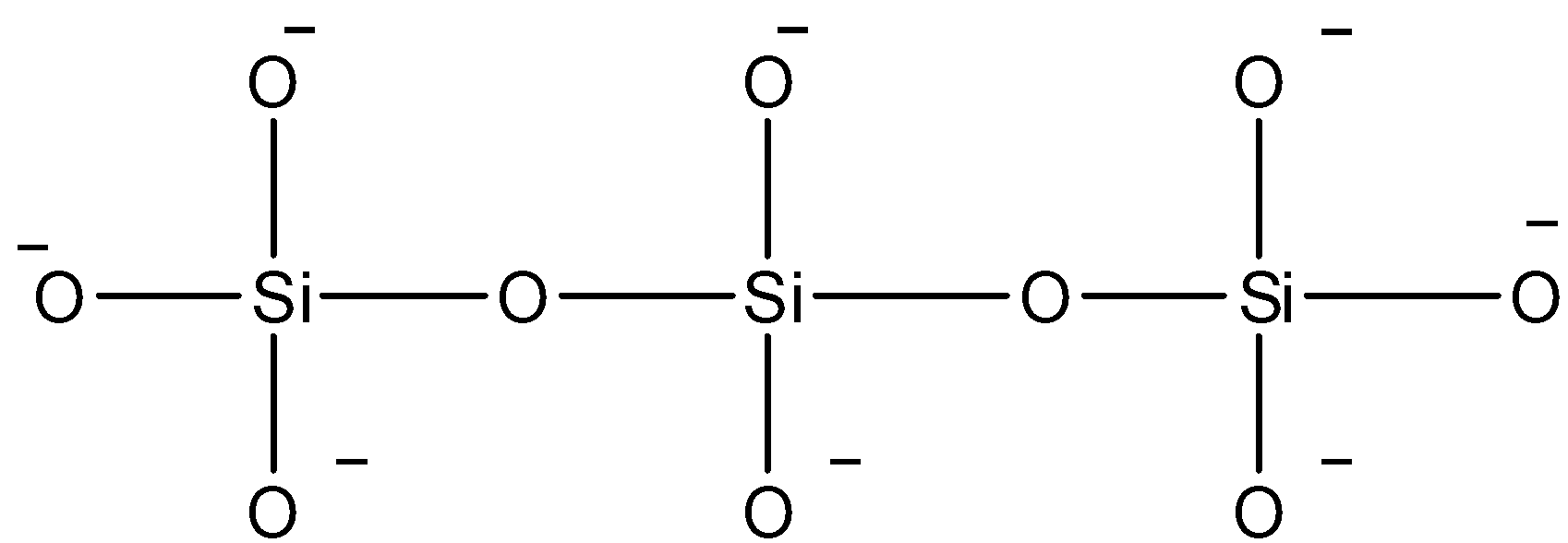
This is how the three \[Si{O_4}^{4 - }\] tetrahedral shares corners with adjacent tetrahedral. When we see at this molecule there are three silicon atoms bonded between two oxygen atoms and each silicon atom is carrying oxygen ion, thus the formula so formed is \[S{i_3}{O_{10}}^{8 - }\] . So we can conclude that the charge on this silicate ion is $ - 8$ .
From the above solved answer it is concluded that option B) is the correct option.
Note:
We have to know that the silicate is a family of atoms which is composed of silicon and oxygen atoms. It is anionic in nature as it carries negative charge on it. The general formula of silicates can be represented as\[{\left[ {Si{O^{\left( {4 - 2x} \right) - }}_{4 - x}} \right]_n}\],where \[0{\text{ }} \leqslant x < {\text{ }}2\].
The silicates include many classes like orthosilicates, pyrosilicates depending upon the changing values of \[x\] in the above equation.
Complete answer:
We have to know that the kinoite mineral has a molecular formula \[C{a_2}C{u_2}S{i_3}{O_8}{(OH)_4}\] it is a bluish colored copper silicate mineral. The crystal structure of this mineral is monoclinic crystal. It is lustrous in appearance and also somewhat transparent.
Now solving the question given it is given that kyanite mineral is a chain of three \[Si{O_4}^{4 - }\] tetrahedral that share corners with adjacent tetrahedral, thus first we need to draw what is asked that makes it easy to answer this question:

This is how the three \[Si{O_4}^{4 - }\] tetrahedral shares corners with adjacent tetrahedral. When we see at this molecule there are three silicon atoms bonded between two oxygen atoms and each silicon atom is carrying oxygen ion, thus the formula so formed is \[S{i_3}{O_{10}}^{8 - }\] . So we can conclude that the charge on this silicate ion is $ - 8$ .
From the above solved answer it is concluded that option B) is the correct option.
Note:
We have to know that the silicate is a family of atoms which is composed of silicon and oxygen atoms. It is anionic in nature as it carries negative charge on it. The general formula of silicates can be represented as\[{\left[ {Si{O^{\left( {4 - 2x} \right) - }}_{4 - x}} \right]_n}\],where \[0{\text{ }} \leqslant x < {\text{ }}2\].
The silicates include many classes like orthosilicates, pyrosilicates depending upon the changing values of \[x\] in the above equation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




