
The shapes $PCl_{4}^{+},PCl_{4}^{-}$ and $AsC{{l}_{5}}$ are respectively:
A. square planar, tetrahedral and see-saw
B. tetrahedral, see-saw and trigonal bipyramidal
C. tetrahedral, square planar and pentagonal bipyramidal
D. trigonal bipyramidal, tetrahedral and square pyramidal
Answer
577.5k+ views
Hint: Hybridization is defined as the combination of two or more atomic orbitals, that results in the formation of a new orbital, which is also known as hybrid orbital. Geometry of a molecule tells us about the shape of a molecule. VSEPR theory is used to predict the geometry of a molecule with the help of an electron pair surrounding the central atoms.
Complete step by step answer:
1. $PCl_{4}^{+}$

This $PCl_{4}^{+}$ shows tetrahedral geometry. It contains a central atom with four substituents at the corner. The tetrahedral structure has $109.5{}^\circ $ angle.
2. $PCl_{4}^{-}$
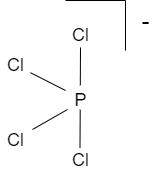
$PCl_{4}^{-}$ shows see-saw structure. There are four bonds to a central atom. In this type of structure, two types of ligands are used, axial and equatorial. The axial pair has a bond angle of $180{}^\circ $ . the angle between the axial and equatorial ligands is around $90{}^\circ $ .
3.$AsC{{l}_{5}}$

$AsC{{l}_{5}}$ shows trigonal bipyramidal geometry. There is one atom at the center and five more atoms at the corner. In this structure, the axial structure is much more crowded because these axial atoms have three neighboring equatorial atoms at a bond angle of $90{}^\circ $ , whereas in the equatorial atom, there are only two neighboring axial atoms at a bond angle of $90{}^\circ $ .
So, the correct answer is Option B .
Note: On the basis of bond angle and bond length, we can specify the geometry of the molecule.To determine the number of electrons in valence shell, lewis structure of a molecule is made. The number of electron pairs will determine the geometry of a molecule.The electron pair orient themselves in such a way that they minimizes the electron electron repulsion but increases the distance between them. There are some of the exceptions of VSEPR theory, like lithium oxide has linear geometry rather than bent .
Complete step by step answer:
1. $PCl_{4}^{+}$

This $PCl_{4}^{+}$ shows tetrahedral geometry. It contains a central atom with four substituents at the corner. The tetrahedral structure has $109.5{}^\circ $ angle.
2. $PCl_{4}^{-}$

$PCl_{4}^{-}$ shows see-saw structure. There are four bonds to a central atom. In this type of structure, two types of ligands are used, axial and equatorial. The axial pair has a bond angle of $180{}^\circ $ . the angle between the axial and equatorial ligands is around $90{}^\circ $ .
3.$AsC{{l}_{5}}$

$AsC{{l}_{5}}$ shows trigonal bipyramidal geometry. There is one atom at the center and five more atoms at the corner. In this structure, the axial structure is much more crowded because these axial atoms have three neighboring equatorial atoms at a bond angle of $90{}^\circ $ , whereas in the equatorial atom, there are only two neighboring axial atoms at a bond angle of $90{}^\circ $ .
So, the correct answer is Option B .
Note: On the basis of bond angle and bond length, we can specify the geometry of the molecule.To determine the number of electrons in valence shell, lewis structure of a molecule is made. The number of electron pairs will determine the geometry of a molecule.The electron pair orient themselves in such a way that they minimizes the electron electron repulsion but increases the distance between them. There are some of the exceptions of VSEPR theory, like lithium oxide has linear geometry rather than bent .
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




