
The shape of $\text{XeF}_{\text{5}}^{-}$ will be:
A) square pyramidal
B) Trigonal bipyramidal
C) Planar
D) Pentagonal bipyramidal
Answer
584.4k+ views
Hint: The valence shell electron pair repulsion (VSEPR) theory is widely used to determine the shape or geometry of a molecule. In $\text{XeF}_{\text{5}}^{-}$ , the 5 bond pairs and two lone pairs are arranged to give the basic shape of a bipyramidal pentagonal. However, the exact shape can be determined by considering the electrostatic repulsion between lone pairs and bond pairs.
Complete step by step answer:
The xenon is a group of 18 elements. The electronic configuration of xenon is as follows:
$\text{Xe = }\left[ \text{Kr} \right]\text{ 4}{{\text{d}}^{\text{10}}}\text{ 5}{{\text{s}}^{\text{2}}}\text{ 5}{{\text{p}}^{\text{6}}}$
The valence shell of xenon contains 2 electrons in the $\text{ 5s}$ and 6 electrons in the $\text{ 5p}$ orbital. Therefore there is a total of 8 valence electron in $\text{ Xe}$.In $\text{XeF}_{\text{5}}^{-}$, the five fluorine atoms donate one electron to the xenon. The $\text{XeF}_{\text{5}}^{-}$ have a negative charge on it thus adding one extra electron to the electron count. Therefore total electron count associated with the $\text{XeF}_{\text{5}}^{-}$ is,
\[\begin{align}
& \text{Total electron in XeF}_{\text{5}}^{-}\text{=Valence electron of Xe + 5 }\!\!\times\!\!\text{ 1}{{\text{e}}^{\text{-}}}\text{ from F + Electron from negative charge} \\
& \text{ = 8 + 5 + 1} \\
& \therefore \text{Total electron in XeF}_{\text{5}}^{-}\text{=14} \\
\end{align}\]
Since each electron pair contains the two electrons thus divide the total number of electrons by the 2 to get the total number of electron pairs.
$\text{ Total number of electron pair = }\dfrac{\text{14 }{{\text{e}}^{\text{-}}}}{\text{2}}\text{ = 7 pairs}$
Here, the $\text{XeF}_{\text{5}}^{-}$ contains the 7 electron pair therefore the possible shape must contain the 7 atoms or lone pair surrounding the central xenon atom. The structure can contain the 5 groups in the axial plane surrounding the xenon atom and two groups can be at the equatorial position. Therefore the possible angle in between the 5 groups in the axial plane is,
$\dfrac{{{360}^{0}}}{5}=\text{ 7}{{\text{2}}^{\text{0}}}$
Thus, the 5 groups are arranged in pentagonal and two bond pairs are at the equatorial position making an angle of ${{90}^{0}}$ with the planar pentagonal shape. Therefore, the possible geometry $\text{XeF}_{\text{5}}^{-}$ is pentagonal bipyramidal.
We have determined the basic shape of the molecule but the adjustments are made based on electrostatic repulsion between the bonding and lone pairs. The repulsion order is
\[\text{ lone pair}-\text{lone pair lone pair}-\text{bonding pair bonding pair}-\text{bonding pair}\]
The lone pairs on average are closer to the nucleus compared to the bonding pair and therefore the lone pairs repel more strongly than the bonding pair.
For $\text{XeF}_{\text{5}}^{-}$ , the parent shape is bipyramidal pentagonal and the structure has two lone pairs. We know that the lone pair lone pair repels each other and destabilizes the molecule. However, the lone pairs can be arranged in such a way that they can experience the minimum repulsion. The lone pairs are arranged opposite to each other making an angle of ${{180}^{0}}$ to minimize the lone pair-lone pair repulsion.
Therefore, the $\text{XeF}_{\text{5}}^{-}$ is a planar structure. The structure of $\text{XeF}_{\text{5}}^{-}$ as shown below,
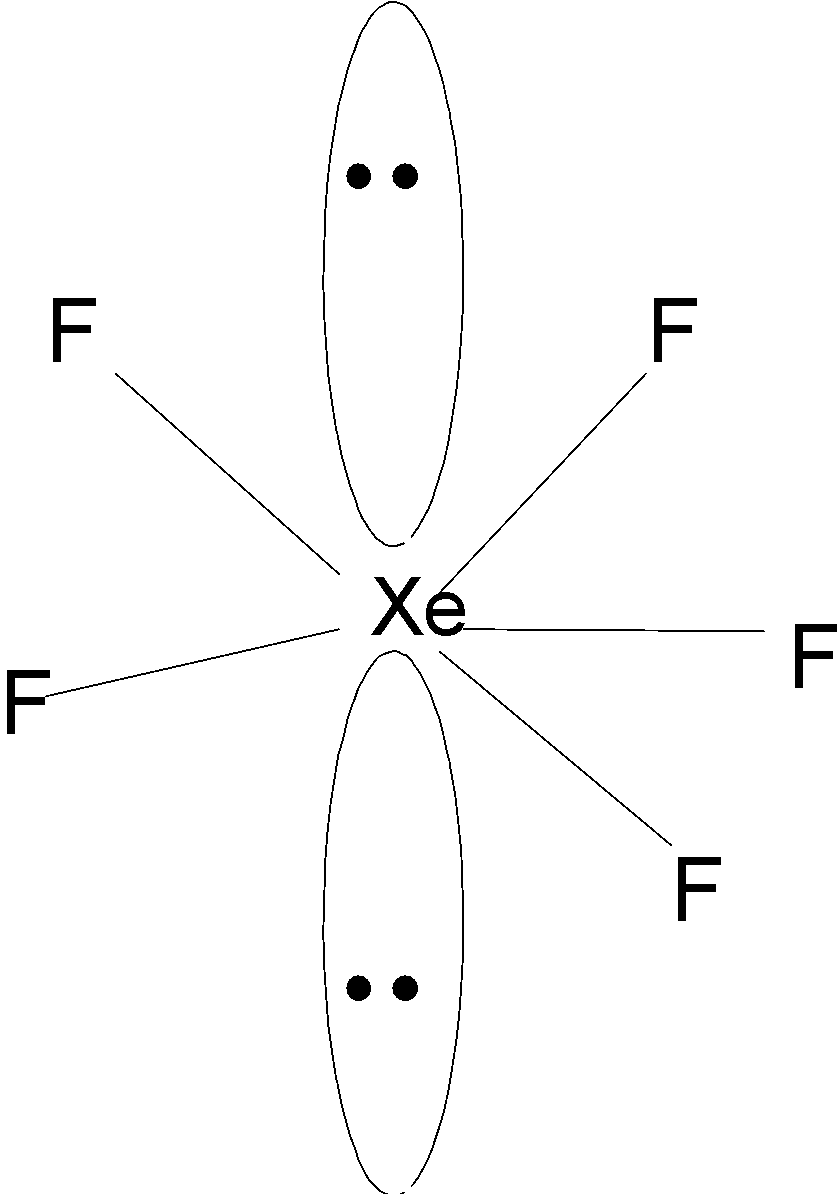
Hence, (D) is the correct option.
Note: we can directly use a formula to find the number of electron pairs surrounding the central atom.
$\text{Number of electron pair =}\dfrac{\text{1}}{\text{2}}\left[ \text{V+N-C+A} \right]$
Where,
V is the number of valence electrons present in the central atom
N is the number of monovalent atoms bonded to the central atom
C is a charge of the cation
A is a charge of the anion
For $\text{XeF}_{\text{5}}^{-}$,
$\begin{align}
& \text{Number of electron pair =}\dfrac{\text{1}}{\text{2}}\left[ \text{V+N-C+A} \right] \\
& \text{ =}\dfrac{\text{1}}{\text{2}}\left[ \text{8+5-0+1} \right] \\
& \text{ =}\dfrac{\text{1}}{\text{2}}\left[ 14 \right] \\
& \therefore \text{Number of electron pair = 7} \\
\end{align}$
Complete step by step answer:
The xenon is a group of 18 elements. The electronic configuration of xenon is as follows:
$\text{Xe = }\left[ \text{Kr} \right]\text{ 4}{{\text{d}}^{\text{10}}}\text{ 5}{{\text{s}}^{\text{2}}}\text{ 5}{{\text{p}}^{\text{6}}}$
The valence shell of xenon contains 2 electrons in the $\text{ 5s}$ and 6 electrons in the $\text{ 5p}$ orbital. Therefore there is a total of 8 valence electron in $\text{ Xe}$.In $\text{XeF}_{\text{5}}^{-}$, the five fluorine atoms donate one electron to the xenon. The $\text{XeF}_{\text{5}}^{-}$ have a negative charge on it thus adding one extra electron to the electron count. Therefore total electron count associated with the $\text{XeF}_{\text{5}}^{-}$ is,
\[\begin{align}
& \text{Total electron in XeF}_{\text{5}}^{-}\text{=Valence electron of Xe + 5 }\!\!\times\!\!\text{ 1}{{\text{e}}^{\text{-}}}\text{ from F + Electron from negative charge} \\
& \text{ = 8 + 5 + 1} \\
& \therefore \text{Total electron in XeF}_{\text{5}}^{-}\text{=14} \\
\end{align}\]
Since each electron pair contains the two electrons thus divide the total number of electrons by the 2 to get the total number of electron pairs.
$\text{ Total number of electron pair = }\dfrac{\text{14 }{{\text{e}}^{\text{-}}}}{\text{2}}\text{ = 7 pairs}$
Here, the $\text{XeF}_{\text{5}}^{-}$ contains the 7 electron pair therefore the possible shape must contain the 7 atoms or lone pair surrounding the central xenon atom. The structure can contain the 5 groups in the axial plane surrounding the xenon atom and two groups can be at the equatorial position. Therefore the possible angle in between the 5 groups in the axial plane is,
$\dfrac{{{360}^{0}}}{5}=\text{ 7}{{\text{2}}^{\text{0}}}$
Thus, the 5 groups are arranged in pentagonal and two bond pairs are at the equatorial position making an angle of ${{90}^{0}}$ with the planar pentagonal shape. Therefore, the possible geometry $\text{XeF}_{\text{5}}^{-}$ is pentagonal bipyramidal.
We have determined the basic shape of the molecule but the adjustments are made based on electrostatic repulsion between the bonding and lone pairs. The repulsion order is
\[\text{ lone pair}-\text{lone pair lone pair}-\text{bonding pair bonding pair}-\text{bonding pair}\]
The lone pairs on average are closer to the nucleus compared to the bonding pair and therefore the lone pairs repel more strongly than the bonding pair.
For $\text{XeF}_{\text{5}}^{-}$ , the parent shape is bipyramidal pentagonal and the structure has two lone pairs. We know that the lone pair lone pair repels each other and destabilizes the molecule. However, the lone pairs can be arranged in such a way that they can experience the minimum repulsion. The lone pairs are arranged opposite to each other making an angle of ${{180}^{0}}$ to minimize the lone pair-lone pair repulsion.
Therefore, the $\text{XeF}_{\text{5}}^{-}$ is a planar structure. The structure of $\text{XeF}_{\text{5}}^{-}$ as shown below,

Hence, (D) is the correct option.
Note: we can directly use a formula to find the number of electron pairs surrounding the central atom.
$\text{Number of electron pair =}\dfrac{\text{1}}{\text{2}}\left[ \text{V+N-C+A} \right]$
Where,
V is the number of valence electrons present in the central atom
N is the number of monovalent atoms bonded to the central atom
C is a charge of the cation
A is a charge of the anion
For $\text{XeF}_{\text{5}}^{-}$,
$\begin{align}
& \text{Number of electron pair =}\dfrac{\text{1}}{\text{2}}\left[ \text{V+N-C+A} \right] \\
& \text{ =}\dfrac{\text{1}}{\text{2}}\left[ \text{8+5-0+1} \right] \\
& \text{ =}\dfrac{\text{1}}{\text{2}}\left[ 14 \right] \\
& \therefore \text{Number of electron pair = 7} \\
\end{align}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




