
The shape of $ {\left( {C{H_3}} \right)_3}N $ is pyramidal because-
A. Nitrogen forms three $ {\text{s}}{{\text{p}}^3} $ hybridized σ bonds with carbon atoms of methyl groups and there is one nonbonding electron pair.
B. Nitrogen forms three $ {\text{s}}{{\text{p}}^3} $ hybridized σ bonds with carbon atoms of methyl groups and fourth orbital forms π bond.
C. Nitrogen has five valencies which are arranged in pyramidal shape.
D. The unpaired electron present on nitrogen is delocalized.
Answer
537.3k+ views
Hint :The nitrogen atom has $ 5 $ valence electrons in which three valence electrons form σ bond with the three carbon atoms of the three methyl groups. The lone pair electrons are free.
Complete Step By Step Answer:
Generally $ {\text{s}}{{\text{p}}^3} $ hybridized molecules show tetrahedral structure but $ {\left( {C{H_3}} \right)_3}N $ is pyramidal despite having $ {\text{s}}{{\text{p}}^3} $ hybridization. We know that electronic configuration of nitrogen is $ 1{s^2},2{s^2}2{p^3} $ . Hence, the nitrogen atom has $ 5 $ valence electrons. Nitrogen forms three $ {\text{s}}{{\text{p}}^3} $ hybridized σ bonds with carbon atoms of methyl groups. Now one electron pair is left. This increases the electron density around the nitrogen atom so there is more repulsion between the electrons pairs around it. This results in a maximum bond angle which is less than that of regular tetrahedron .Due to the presence of these lone pair electrons the geometry of the molecule is distorted and it forms pyramidal shape or distorted tetrahedral. It shape is given below-
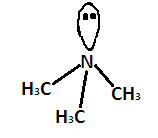
According to VSEPR theory, the shape should be tetrahedral as the electron pair will repel each other and bond angle should be $ {109.5^ \circ } $ .
But the lone pair electrons on nitrogen repel the adjacent bonding pairs more strongly than the bonding pairs repel each other. Due to this the bond angle between the bonding pairs becomes $ {107.3^ \circ } $
The shape changes from tetrahedral to pyramidal.
Hence, Nitrogen forms three $ {\text{s}}{{\text{p}}^3} $ hybridized σ bonds with carbon atoms of methyl groups and there is one nonbonding electron pair.
So option A is correct.
Note :
The structure of trimethylamine $ {\left( {C{H_3}} \right)_3}N $ is similar to the structure of ammonia in which three hybridized orbitals of nitrogen are involved in axial overlap with orbitals of carbon atoms. The lone pair on the nitrogen atom is free and the amine acts as Lewis base.
Complete Step By Step Answer:
Generally $ {\text{s}}{{\text{p}}^3} $ hybridized molecules show tetrahedral structure but $ {\left( {C{H_3}} \right)_3}N $ is pyramidal despite having $ {\text{s}}{{\text{p}}^3} $ hybridization. We know that electronic configuration of nitrogen is $ 1{s^2},2{s^2}2{p^3} $ . Hence, the nitrogen atom has $ 5 $ valence electrons. Nitrogen forms three $ {\text{s}}{{\text{p}}^3} $ hybridized σ bonds with carbon atoms of methyl groups. Now one electron pair is left. This increases the electron density around the nitrogen atom so there is more repulsion between the electrons pairs around it. This results in a maximum bond angle which is less than that of regular tetrahedron .Due to the presence of these lone pair electrons the geometry of the molecule is distorted and it forms pyramidal shape or distorted tetrahedral. It shape is given below-

According to VSEPR theory, the shape should be tetrahedral as the electron pair will repel each other and bond angle should be $ {109.5^ \circ } $ .
But the lone pair electrons on nitrogen repel the adjacent bonding pairs more strongly than the bonding pairs repel each other. Due to this the bond angle between the bonding pairs becomes $ {107.3^ \circ } $
The shape changes from tetrahedral to pyramidal.
Hence, Nitrogen forms three $ {\text{s}}{{\text{p}}^3} $ hybridized σ bonds with carbon atoms of methyl groups and there is one nonbonding electron pair.
So option A is correct.
Note :
The structure of trimethylamine $ {\left( {C{H_3}} \right)_3}N $ is similar to the structure of ammonia in which three hybridized orbitals of nitrogen are involved in axial overlap with orbitals of carbon atoms. The lone pair on the nitrogen atom is free and the amine acts as Lewis base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




