
The shape of ${H_2}O$ molecule is:
A.) Linear
B.) Tetrahedral
C.) V-shape
D.) Trigonal planar
Answer
584.7k+ views
Hint: In water molecules there are two lone pairs on oxygen. According to VSEPR theory, there are repulsions of lone pair-lone pair repulsions, the water molecule tends to acquire bent shape or V-shape.
Complete step by step answer:
The VSEPR theory is used to predict the shapes of molecules. Here, VSEPR is used as an abbreviation for Valence Shell Electron Pair Repulsion Theory. VSEPR theory is based on the assumption that there is a repulsion between the pairs of valence electrons in all atoms, and atoms tend to arrange themselves in a manner such that the electronic repulsion in the valence shell of the atom is minimized.
According to VSEPR theory, the order of repulsion between electron pairs can be given as follows: lone pair-lone pair $ > $ lone pair-bond pair $ > $ Bond pair-bond pair repulsions.
To find structure of a molecule we can use the following formula :
$n = \dfrac{{(V + M - C + A)}}{2}$
Where, $V = $ Number of valence electrons of the central atom
$M = $ Number of monovalent atoms attached to central atom
$C = $ Number of cations
$A = $ number of anions.
Now, for water molecules, the central atom is the oxygen and we know that it has six valence electrons. Thus, $V = 6$. Also, we know that the number of atoms attached to the oxygen in water molecules is two. Hence, $M = 2$. As there are no anion or cation present in water molecules so the value of $A{\text{ and C = 0}}$.
Now by putting these values in equation in above equation we get:
$n = \dfrac{{6 + 2}}{2} = 4$
Therefore, the total lone pairs plus bond pairs in water molecules is four. As we know that oxygen has six valence so it shares two electrons (one with each hydrogen) and there are remaining four electrons which makes the two lone pairs on oxygen.
Hence, a water molecule has two lone pairs and two bond pairs. Due to the repulsion between lone pair- lone pair, it acquires bent shape or V-shape. This shape can be shown as :
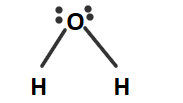
So, the correct answer is Option C .
Note:
Remember that there is a difference between the geometry and shape of a molecule. In case of geometry we consider the lone pairs in it but for finding shape we do not consider lone pairs. Example- Geometry of water is tetrahedral but its shape is V-shape.
Complete step by step answer:
The VSEPR theory is used to predict the shapes of molecules. Here, VSEPR is used as an abbreviation for Valence Shell Electron Pair Repulsion Theory. VSEPR theory is based on the assumption that there is a repulsion between the pairs of valence electrons in all atoms, and atoms tend to arrange themselves in a manner such that the electronic repulsion in the valence shell of the atom is minimized.
According to VSEPR theory, the order of repulsion between electron pairs can be given as follows: lone pair-lone pair $ > $ lone pair-bond pair $ > $ Bond pair-bond pair repulsions.
To find structure of a molecule we can use the following formula :
$n = \dfrac{{(V + M - C + A)}}{2}$
Where, $V = $ Number of valence electrons of the central atom
$M = $ Number of monovalent atoms attached to central atom
$C = $ Number of cations
$A = $ number of anions.
Now, for water molecules, the central atom is the oxygen and we know that it has six valence electrons. Thus, $V = 6$. Also, we know that the number of atoms attached to the oxygen in water molecules is two. Hence, $M = 2$. As there are no anion or cation present in water molecules so the value of $A{\text{ and C = 0}}$.
Now by putting these values in equation in above equation we get:
$n = \dfrac{{6 + 2}}{2} = 4$
Therefore, the total lone pairs plus bond pairs in water molecules is four. As we know that oxygen has six valence so it shares two electrons (one with each hydrogen) and there are remaining four electrons which makes the two lone pairs on oxygen.
Hence, a water molecule has two lone pairs and two bond pairs. Due to the repulsion between lone pair- lone pair, it acquires bent shape or V-shape. This shape can be shown as :

So, the correct answer is Option C .
Note:
Remember that there is a difference between the geometry and shape of a molecule. In case of geometry we consider the lone pairs in it but for finding shape we do not consider lone pairs. Example- Geometry of water is tetrahedral but its shape is V-shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




