
The resultant dipole moment $\mu $ of two components of NOF and $N{O_2}F$ is 1.81 D and 0.47 D respectively. Which dipole moment do you predict?
A) 1.81 D for $N{O_2}F$ and 0.47 for NOF
B) 0.47 for $N{O_2}F$ and 1.81 for NOF
C) For both $N{O_2}F$ and NOF, dipole moment ($\mu $)is 1.81 D
D) For both $N{O_2}F$ and NOF, dipole moment ($\mu $) is 0.47D
Answer
591.3k+ views
Hint: To predict the dipole moment of NOF and $N{O_2}F$, you must know their structures. NOF is bent in shape while $N{O_2}F$ is trigonal planar in shape. A NOF molecule contains a lone pair on a nitrogen atom.
Complete step by step answer:
Structure of NOF is as shown below:
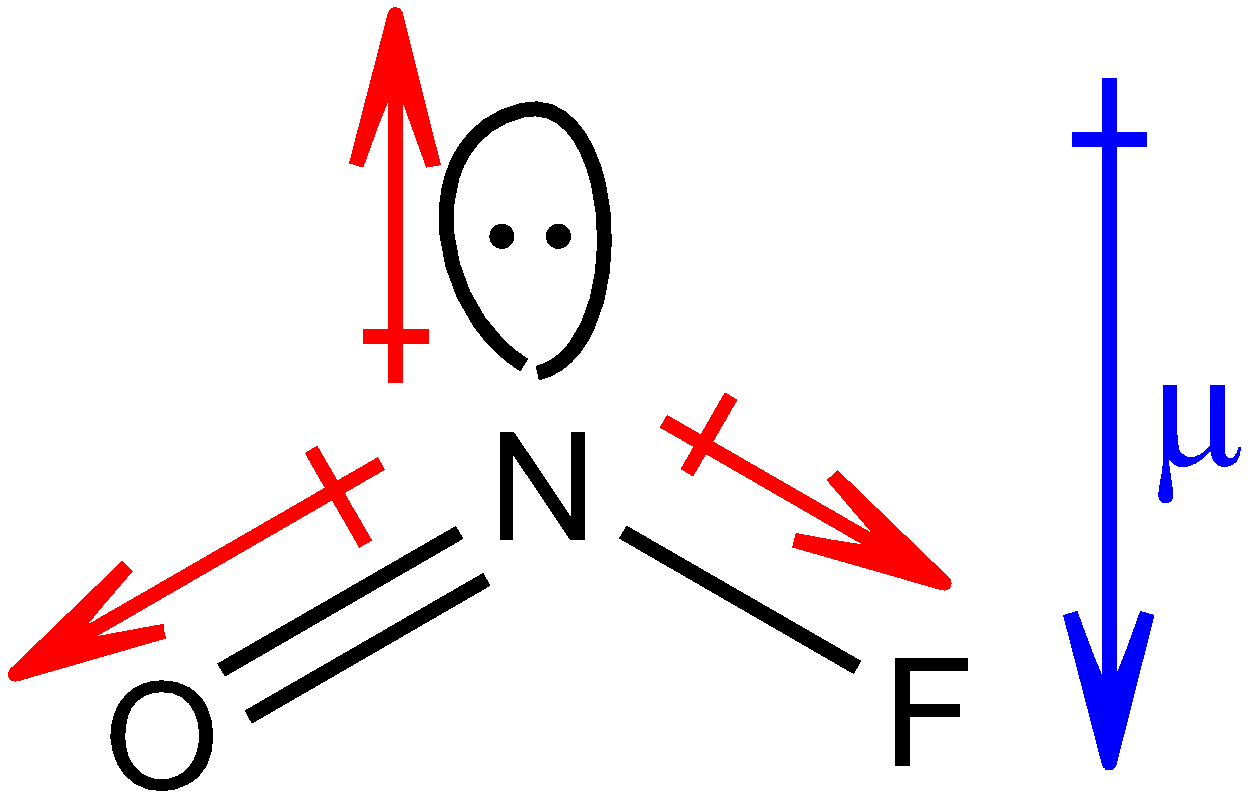
The red colour arrows show the resultant dipole moment between the respective two elements. The head of the arrow of dipole moment always points towards the more electronegative atom. The blue colour arrow shows the net resultant dipole moment (µ) in the molecule.
Structure of $N{O_2}F$ is as shown below:
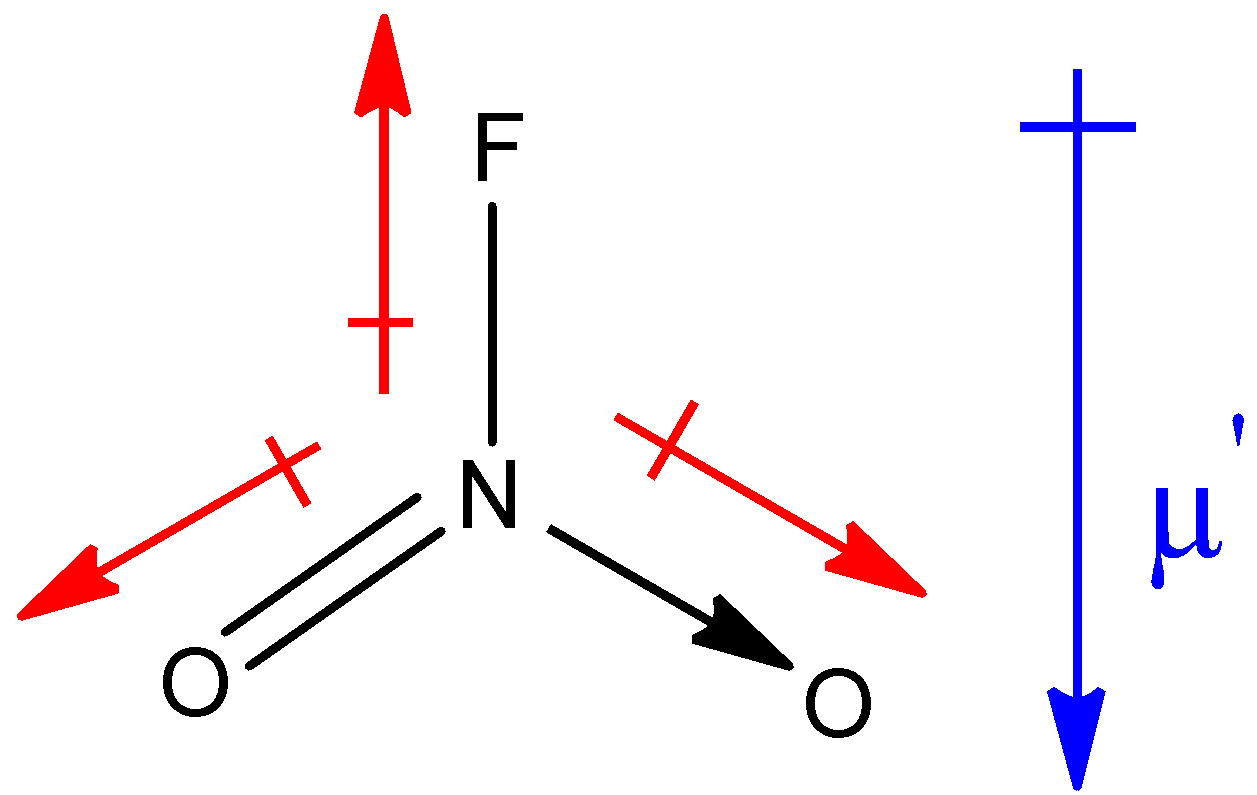
Similarly, in the above structure, the red colour arrows show the resultant dipole moment between the respective two elements and the blue colour arrow shows the net resultant dipole moment (µ’) in the molecule.
In both the molecules, net resultant dipole moment is in the same direction. But you must know that the dipole moment of the lone pair is always greater than the dipole between the bonds of two shared pairs of electrons. Therefore, the net resultant dipole moment of NOF molecule will have greater value than that of $N{O_2}F$ molecule because of the presence of a lone pair in the NOF molecule, thus, µ > µ’.
Hence, the dipole moment for $N{O_2}F$ will be 0.47 D and for NOF, it will be 1.81 D.
So, the correct answer is “Option B”.
Note: A dipole moment is the product of the magnitude of separated charges and the distance of separation between them. Its SI unit is Debye, D. Dipole moment is denoted by an arrow with cross on the positive centre or the less electronegative atom and arrow head on the negative centre or the more electronegative atom. If a molecule has a dipole moment, we call it polar. Dipole moment is zero for non-polar compounds.
Complete step by step answer:
Structure of NOF is as shown below:

The red colour arrows show the resultant dipole moment between the respective two elements. The head of the arrow of dipole moment always points towards the more electronegative atom. The blue colour arrow shows the net resultant dipole moment (µ) in the molecule.
Structure of $N{O_2}F$ is as shown below:

Similarly, in the above structure, the red colour arrows show the resultant dipole moment between the respective two elements and the blue colour arrow shows the net resultant dipole moment (µ’) in the molecule.
In both the molecules, net resultant dipole moment is in the same direction. But you must know that the dipole moment of the lone pair is always greater than the dipole between the bonds of two shared pairs of electrons. Therefore, the net resultant dipole moment of NOF molecule will have greater value than that of $N{O_2}F$ molecule because of the presence of a lone pair in the NOF molecule, thus, µ > µ’.
Hence, the dipole moment for $N{O_2}F$ will be 0.47 D and for NOF, it will be 1.81 D.
So, the correct answer is “Option B”.
Note: A dipole moment is the product of the magnitude of separated charges and the distance of separation between them. Its SI unit is Debye, D. Dipole moment is denoted by an arrow with cross on the positive centre or the less electronegative atom and arrow head on the negative centre or the more electronegative atom. If a molecule has a dipole moment, we call it polar. Dipole moment is zero for non-polar compounds.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




