
The reaction of propene with ${{H}_{3}}{{O}^{+}}$will proceed with which of the following intermediate?
A.
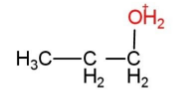
B.
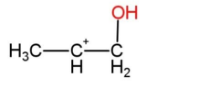
C.

D.
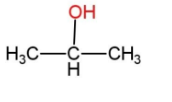




Answer
533.4k+ views
Hint:The reaction where any alkene is subjected to the addition of water molecules is known as hydration reaction. The hydration of alkenes yields alcohol. This reaction is based on markovnikov rule, and is a type of electrophilic addition reaction.
Complete step-by-step answer:The reaction where alkenes react with water is known as hydration of alkenes. It follows the Markovnikov rule that states that the negative part of the addendum goes to that carbon of the carbon- carbon double bond which has less number of hydrogen atoms.
Therefore, a reactive intermediate is formed in these types of reactions where the water molecule gets attached to the carbon according to the markovnikov rule. The reaction happens in 3 steps, in the first step the protonation occurs, then the water molecule gets attached, and in the last step deprotonation occurs to form an alcohol.
For propene, the hydration occurs as follows,

A carbocation is formed after protonation, and then the electrophile of water attacks this carbocation, forming an intermediate, which on deprotonation forms 2-propanol.
As the reaction is according to Markovnikov rule, so the intermediate is option C, that has water attached with carbon that has less hydrogen atoms.
Hence, option C is correct.
Note:Addition of water to alkenes happens in the similar way as that of addition of halogen acids, as water and halogen acids both have the same type of H-X bond which have the ability to donate protons.
Complete step-by-step answer:The reaction where alkenes react with water is known as hydration of alkenes. It follows the Markovnikov rule that states that the negative part of the addendum goes to that carbon of the carbon- carbon double bond which has less number of hydrogen atoms.
Therefore, a reactive intermediate is formed in these types of reactions where the water molecule gets attached to the carbon according to the markovnikov rule. The reaction happens in 3 steps, in the first step the protonation occurs, then the water molecule gets attached, and in the last step deprotonation occurs to form an alcohol.
For propene, the hydration occurs as follows,

A carbocation is formed after protonation, and then the electrophile of water attacks this carbocation, forming an intermediate, which on deprotonation forms 2-propanol.
As the reaction is according to Markovnikov rule, so the intermediate is option C, that has water attached with carbon that has less hydrogen atoms.
Hence, option C is correct.
Note:Addition of water to alkenes happens in the similar way as that of addition of halogen acids, as water and halogen acids both have the same type of H-X bond which have the ability to donate protons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




