
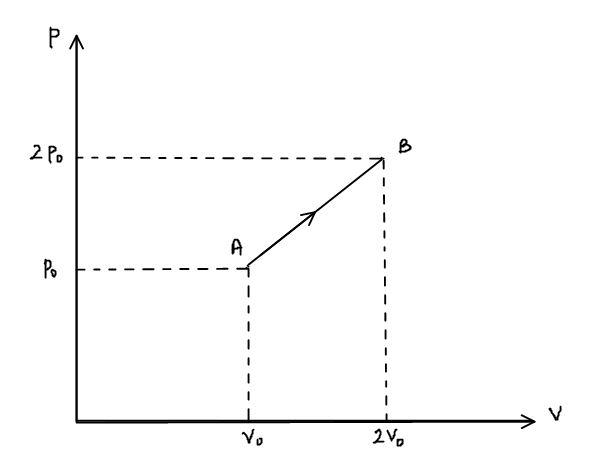
The p-v diagram of 2g of helium gas for a certain process A→B is shown in the figure below. What is the heat given to the gas during the process A→B?
A. $4{p_ \circ }{V_ \circ }$
B. $6{p_ \circ }{V_ \circ }$
C. $4.5{p_ \circ }{V_ \circ }$
D. $2{p_ \circ }{V_ \circ }$

Answer
588.9k+ views
Hint: In such types of questions, students should observe which type of process the graph is following and also the initial and final coordinates of the process given in the graph.
Complete Step by Step Answer:
Step 1:
As the gas is helium and the degree of freedom for helium gas is 3, i.e. $F = 3$.
Here, F is a degree of freedom.
Step 2:
Due to change in process, there will be change in internal energy of the gas, so let’s calculate the change in internal energy of the gas,
The change in internal energy of the gas is given by,
$\Delta U = \dfrac{F}{2} \cdot \Delta \left( {P \cdot V} \right)$
Here, $\Delta \left( {P \cdot V} \right)$ is \[{P_2} \cdot {V_2} - {P_1} \cdot {V_1}\] which can be calculated from the given graph.
And F is the degree of freedom and $\Delta U$ is a change in internal energy of the gas.
Substituting the value of $\Delta \left( {P \cdot V} \right)$, the relation becomes,
\[\Delta U = \dfrac{F}{2} \cdot \left( {{P_2} \cdot {V_2} - {P_1} \cdot {V_1}} \right)\]
Step 3:
The value of ${P_2} = 2{p_ \circ }$, ${V_2} = 2{V_ \circ }$, ${P_1} = {p_ \circ }$ and ${V_1} = {V_ \circ }$ substituting these values in the above relation,
We get,
\[
\Delta U = \dfrac{3}{2} \cdot \left( {4{p_ \circ }{V_ \circ } - {p_ \circ }{V_ \circ }} \right) \\
\Delta U = \dfrac{3}{2} \cdot \left( {3{p_ \circ }{V_ \circ }} \right) \\
\Delta U = \dfrac{9}{2} \cdot \left( {{p_ \circ }{V_ \circ }} \right) \\
\] ………eq.1
So, the change in internal energy is equals to \[\Delta U = \dfrac{9}{2} \cdot \left( {{p_ \circ }{V_ \circ }} \right)\].
Step 4:
The area of pressure-volume graph is equal to the work done by the gas,
Therefore, the area of trapezium is given by,
$
W = \dfrac{1}{2} \cdot \left( {2{p_ \circ } + {p_ \circ }} \right) \cdot {V_ \circ } \\
W = \dfrac{1}{2} \cdot 3{p_ \circ } \cdot {V_ \circ } \\
W = \dfrac{3}{2} \cdot {p_ \circ } \cdot {V_ \circ } \\
$………eq.2
So, the work done by the gas is given by, $W = \dfrac{3}{2} \cdot {p_ \circ } \cdot {V_ \circ }$,
Step 5:
The first law of thermodynamics says,
$\Delta Q = W + \Delta U$
Where,
$\Delta Q$ = Change in heat transfer of gas.
W = Work done by the gas.
$\Delta U$=Change in internal energy of the gas.
Putting the value of the work done by gas and change in internal energy of the gas in the above relation from equation 1 and equation 2.
We get,
\[
\Delta Q = \dfrac{3}{2} \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) + \dfrac{9}{2} \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) \\
\Delta Q = \dfrac{{12}}{2} \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) \\
\Delta Q = 6 \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) \\
\]
$\therefore$ the heat given to the gas during the process A→B is $6{p_ \circ }{V_ \circ }$. so, the correct option is (B).
Note:
- The degree of freedom of the helium is given as 3, which means translation motion is possible only in three directions.
- The area of the pressure-volume graph gives work done by the gas. Don’t get confused while taking the area of the trapezium which is formed by the process A $\to$ B.
- The area of trapezium is taken by using the formula of area of trapezium which is Area=$\dfrac{1}{2} \times $(Sum of parallel sides)$\times $(distance between the parallel sides).
Complete Step by Step Answer:
Step 1:
As the gas is helium and the degree of freedom for helium gas is 3, i.e. $F = 3$.
Here, F is a degree of freedom.
Step 2:
Due to change in process, there will be change in internal energy of the gas, so let’s calculate the change in internal energy of the gas,
The change in internal energy of the gas is given by,
$\Delta U = \dfrac{F}{2} \cdot \Delta \left( {P \cdot V} \right)$
Here, $\Delta \left( {P \cdot V} \right)$ is \[{P_2} \cdot {V_2} - {P_1} \cdot {V_1}\] which can be calculated from the given graph.
And F is the degree of freedom and $\Delta U$ is a change in internal energy of the gas.
Substituting the value of $\Delta \left( {P \cdot V} \right)$, the relation becomes,
\[\Delta U = \dfrac{F}{2} \cdot \left( {{P_2} \cdot {V_2} - {P_1} \cdot {V_1}} \right)\]
Step 3:
The value of ${P_2} = 2{p_ \circ }$, ${V_2} = 2{V_ \circ }$, ${P_1} = {p_ \circ }$ and ${V_1} = {V_ \circ }$ substituting these values in the above relation,
We get,
\[
\Delta U = \dfrac{3}{2} \cdot \left( {4{p_ \circ }{V_ \circ } - {p_ \circ }{V_ \circ }} \right) \\
\Delta U = \dfrac{3}{2} \cdot \left( {3{p_ \circ }{V_ \circ }} \right) \\
\Delta U = \dfrac{9}{2} \cdot \left( {{p_ \circ }{V_ \circ }} \right) \\
\] ………eq.1
So, the change in internal energy is equals to \[\Delta U = \dfrac{9}{2} \cdot \left( {{p_ \circ }{V_ \circ }} \right)\].
Step 4:
The area of pressure-volume graph is equal to the work done by the gas,
Therefore, the area of trapezium is given by,
$
W = \dfrac{1}{2} \cdot \left( {2{p_ \circ } + {p_ \circ }} \right) \cdot {V_ \circ } \\
W = \dfrac{1}{2} \cdot 3{p_ \circ } \cdot {V_ \circ } \\
W = \dfrac{3}{2} \cdot {p_ \circ } \cdot {V_ \circ } \\
$………eq.2
So, the work done by the gas is given by, $W = \dfrac{3}{2} \cdot {p_ \circ } \cdot {V_ \circ }$,
Step 5:
The first law of thermodynamics says,
$\Delta Q = W + \Delta U$
Where,
$\Delta Q$ = Change in heat transfer of gas.
W = Work done by the gas.
$\Delta U$=Change in internal energy of the gas.
Putting the value of the work done by gas and change in internal energy of the gas in the above relation from equation 1 and equation 2.
We get,
\[
\Delta Q = \dfrac{3}{2} \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) + \dfrac{9}{2} \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) \\
\Delta Q = \dfrac{{12}}{2} \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) \\
\Delta Q = 6 \cdot \left( {{p_ \circ } \cdot {V_ \circ }} \right) \\
\]
$\therefore$ the heat given to the gas during the process A→B is $6{p_ \circ }{V_ \circ }$. so, the correct option is (B).
Note:
- The degree of freedom of the helium is given as 3, which means translation motion is possible only in three directions.
- The area of the pressure-volume graph gives work done by the gas. Don’t get confused while taking the area of the trapezium which is formed by the process A $\to$ B.
- The area of trapezium is taken by using the formula of area of trapezium which is Area=$\dfrac{1}{2} \times $(Sum of parallel sides)$\times $(distance between the parallel sides).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




