
The products (A), (B) and (C) are:
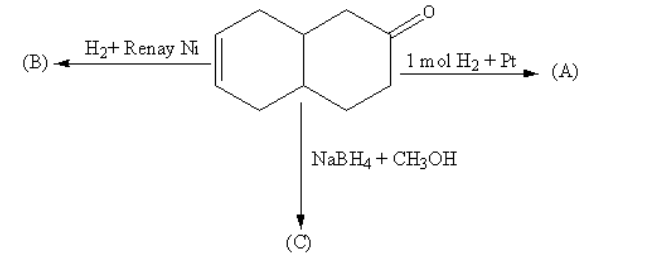
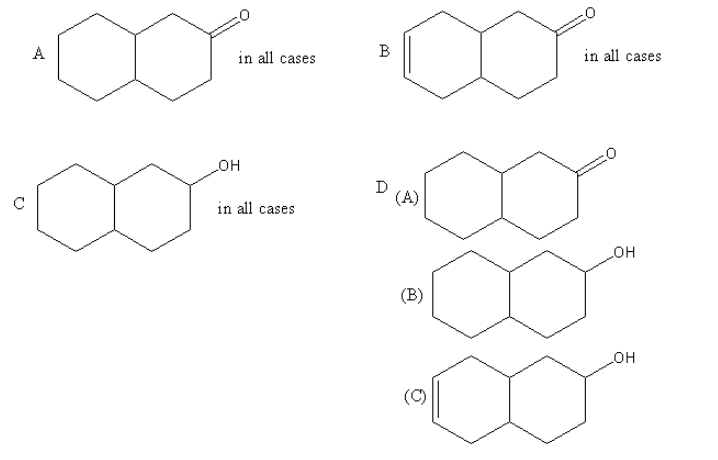
Which of the following options is correct?


Answer
573.9k+ views
Hint: Platinum and Raney nickel are the catalyst. Platinum with hydrogen is used for hydrogenation. Raney nickel with hydrogen is used to reduce unsaturated compounds. Sodium borohydride with methanol is used to reduce carbonyls selectively.
Complete Step by step answer:
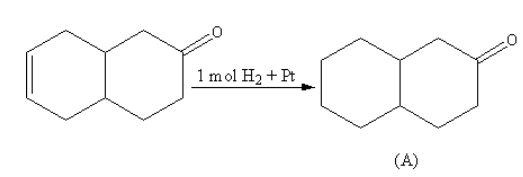
Hydrogen in presence of platinum is used for the reduction of alkenes to form alkanes. The platinum with hydrogen reduces alkene only, not carbonyl, so product A will have carbonyl at one ring which will be attached to the cyclohexane ring.
The reaction of the compound with hydrogen in presence of platinum is as follows:
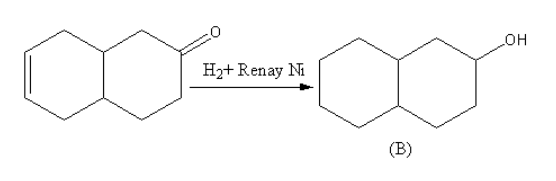
Hydrogen in presence of Raney nickel is used for the reduction of unsaturated compounds to form saturated compounds. The Raney nickel is used as a catalyst. The Raney nickel with hydrogen reduces almost all unsaturated compounds containing alkene, alkyne, carbonyls, hydrazine and nitro compounds, so the product B will be phenol attached with cyclohexane.
The reaction of the compound with hydrogen in presence of Raney nickel is as follows:
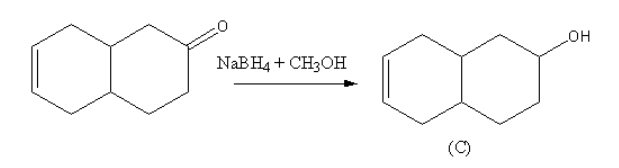
Sodium borohydride in presence of methanol is used for the reduction of carbonyls. The Sodium borohydride in presence of methanol reduces aldehyde and ketone to alcohol so, the product C will be phenol attached with cyclohexene.
The reaction of the compound with sodium borohydride in presence of methanol is as follows:
Hence the correct option is (D).
Note: Hydrogen with platinum metal, hydrogen with Raney nickel, and sodium borohydride with methanol all are reducing agents. Hydrogen with platinum metal reduces only alkenes and alkynes. Hydrogen with Raney nickel reduces all unsaturated compounds. Sodium borohydride with methanol is a chemoselective reagent. It selectively reduces carbonyls. It does not reduce esters, amides and carboxylic acids.
Complete Step by step answer:
Hydrogen in presence of platinum is used for the reduction of alkenes to form alkanes. The platinum with hydrogen reduces alkene only, not carbonyl, so product A will have carbonyl at one ring which will be attached to the cyclohexane ring.
The reaction of the compound with hydrogen in presence of platinum is as follows:

Hydrogen in presence of Raney nickel is used for the reduction of unsaturated compounds to form saturated compounds. The Raney nickel is used as a catalyst. The Raney nickel with hydrogen reduces almost all unsaturated compounds containing alkene, alkyne, carbonyls, hydrazine and nitro compounds, so the product B will be phenol attached with cyclohexane.
The reaction of the compound with hydrogen in presence of Raney nickel is as follows:

Sodium borohydride in presence of methanol is used for the reduction of carbonyls. The Sodium borohydride in presence of methanol reduces aldehyde and ketone to alcohol so, the product C will be phenol attached with cyclohexene.
The reaction of the compound with sodium borohydride in presence of methanol is as follows:

Hence the correct option is (D).
Note: Hydrogen with platinum metal, hydrogen with Raney nickel, and sodium borohydride with methanol all are reducing agents. Hydrogen with platinum metal reduces only alkenes and alkynes. Hydrogen with Raney nickel reduces all unsaturated compounds. Sodium borohydride with methanol is a chemoselective reagent. It selectively reduces carbonyls. It does not reduce esters, amides and carboxylic acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




