
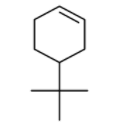
The product of the given reaction is:
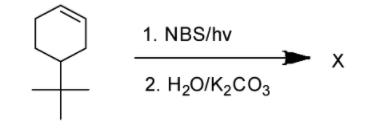
A.
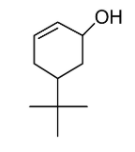
B.
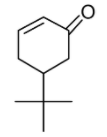
C.
D.





Answer
577.5k+ views
Hint: The NBS i.e. N-Bromosuccinimide is actually a brominating as well as oxidizing agent which is generally used as a source of bromine in the radical reactions (such as allylic bromination) or several electrophilic additions. The chemical formula of NBS is \[{C_4}{H_4}BrN{O_2}\].
Detailed explanation: Allylic bromination is actually the process in which hydrogen on a carbon atom adjacent to a double bond is being replaced. Generally NBS bromination (i.e. NBS is used as reagent in bromination) of substrates like alcohols or amines are followed by the elimination of HBr in the presence of base which ultimately leads to the formation of products of net oxidation which do not contain any incorporated bromine.
In the present case, the substrate is 4-tert-butyl cyclohex-1-ene. Allylic bromination of 4-tert-butyl cyclohex-1-ene using NBS/hv as a reagent yields 3-bromo-5-tert-butyl cyclohex-1-ene. We can say that allylic H atom is being replaced with Br. Now in the next step, hydrolysis of C−Br bond will take place leading to the formation of C−OH bond. The reaction has been demonstrated below:
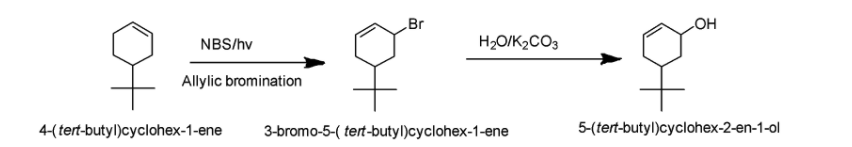
Hence, the correct answer is Option A i.e 5-(tert-butyl)cyclohex-2-en-1-ol.
Note:
NBS is used instead of directly using Br2 in allylic bromination reactions because Br2 tends to react with the double bonds in order to form dibromides. On the other hand, if we use NBS as a substitute for Br2, it supplies a lower concentration of Br2, and thus, bromination of double bonds doesn't compete as much as without NBS.
Detailed explanation: Allylic bromination is actually the process in which hydrogen on a carbon atom adjacent to a double bond is being replaced. Generally NBS bromination (i.e. NBS is used as reagent in bromination) of substrates like alcohols or amines are followed by the elimination of HBr in the presence of base which ultimately leads to the formation of products of net oxidation which do not contain any incorporated bromine.
In the present case, the substrate is 4-tert-butyl cyclohex-1-ene. Allylic bromination of 4-tert-butyl cyclohex-1-ene using NBS/hv as a reagent yields 3-bromo-5-tert-butyl cyclohex-1-ene. We can say that allylic H atom is being replaced with Br. Now in the next step, hydrolysis of C−Br bond will take place leading to the formation of C−OH bond. The reaction has been demonstrated below:

Hence, the correct answer is Option A i.e 5-(tert-butyl)cyclohex-2-en-1-ol.
Note:
NBS is used instead of directly using Br2 in allylic bromination reactions because Br2 tends to react with the double bonds in order to form dibromides. On the other hand, if we use NBS as a substitute for Br2, it supplies a lower concentration of Br2, and thus, bromination of double bonds doesn't compete as much as without NBS.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




