
The product (A) and (B) are respectively:
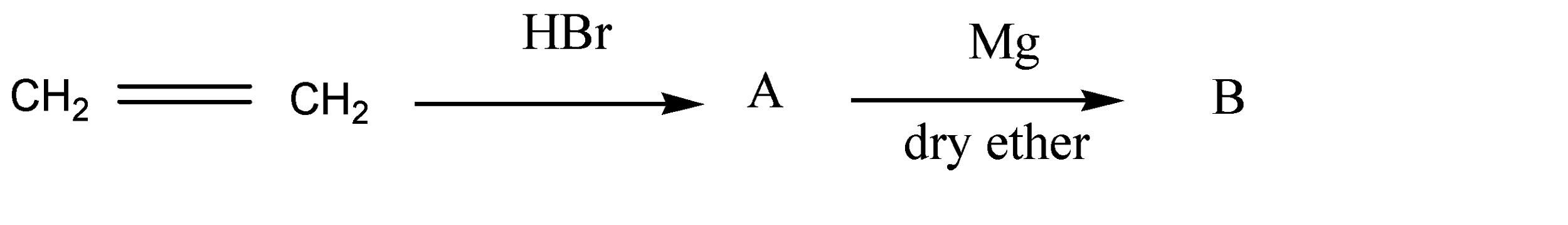

Answer
575.7k+ views
Hint:Ethene is symmetrical alkene which contains two carbon atoms. In presence of HBr, ethene will give bromoethane as the product. This reaction is an additional reaction. In presence of organic peroxide, the halogen adds with the carbon in double bond having greater number of hydrogen atoms. It is anti markovnikov addition. It is called the Kharasch effect.
Complete step by step answer:
As we know ethene has two carbon atoms which are symmetrical alkene. Ethene reacts with HBr and gives ethyl bromide in the first step as a product. The reaction is given below:
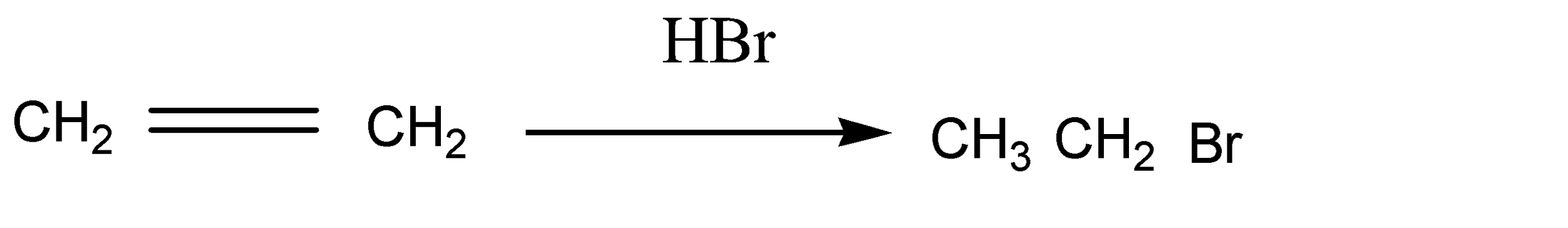
HBr molecules are added across the double bond. Here the anti markovnikov rule is assumed because the molecule is symmetrical around the double bond. Otherwise the number of hydrogen atoms belonging to double bond carbon atoms are equal.
In the next step, bromoethane reacts in the presence of Mg and dry ether and forms a new product. Here the reaction is given below:
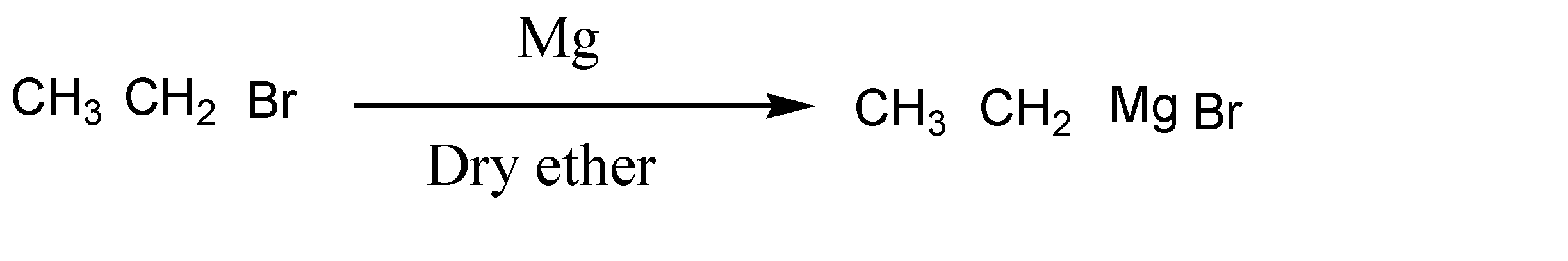
Here bromo ethane reacts with Mg in presence of dry ether and forms ethyl magnesium bromide.
So, the reaction is given as below:
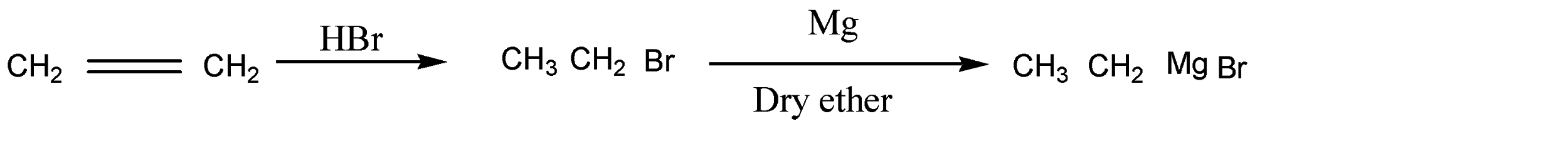
So here product A is bromoethane and product B is ethyl magnesium bromide.
Note: HBr adds to alkenes to create alkyl halides. A good way to think of the reaction is that the pi bond of alkene acts as a weak nucleophilic and reacts with the electrophilic proton with HBr. These are really just two ways to think about the same event. Either way a carbocation intermediate is formed along with the bromide anion during the initial step of reaction.
Complete step by step answer:
As we know ethene has two carbon atoms which are symmetrical alkene. Ethene reacts with HBr and gives ethyl bromide in the first step as a product. The reaction is given below:

HBr molecules are added across the double bond. Here the anti markovnikov rule is assumed because the molecule is symmetrical around the double bond. Otherwise the number of hydrogen atoms belonging to double bond carbon atoms are equal.
In the next step, bromoethane reacts in the presence of Mg and dry ether and forms a new product. Here the reaction is given below:

Here bromo ethane reacts with Mg in presence of dry ether and forms ethyl magnesium bromide.
So, the reaction is given as below:
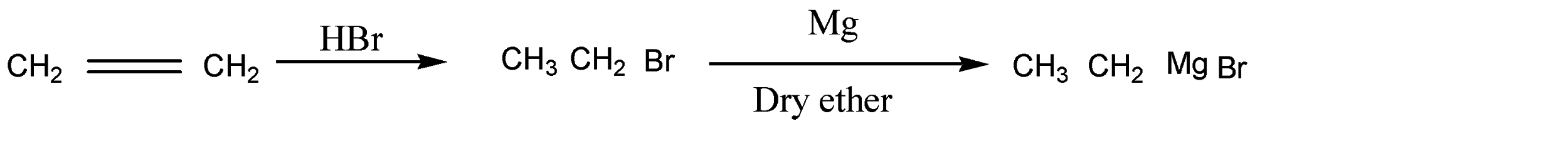
So here product A is bromoethane and product B is ethyl magnesium bromide.
Note: HBr adds to alkenes to create alkyl halides. A good way to think of the reaction is that the pi bond of alkene acts as a weak nucleophilic and reacts with the electrophilic proton with HBr. These are really just two ways to think about the same event. Either way a carbocation intermediate is formed along with the bromide anion during the initial step of reaction.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




