
The potential energy diagram for a reaction $X \to Y$ is given. A and C in the graph corresponds to:
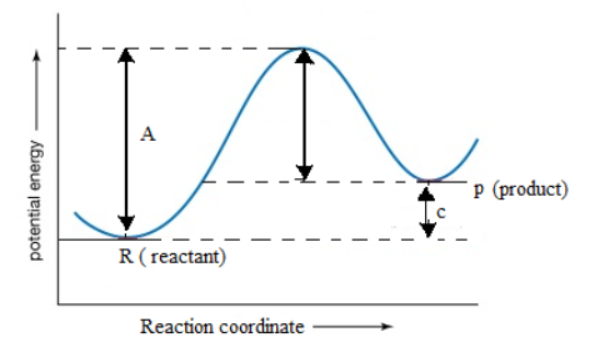
(A) A$ \to $Activation energy, C$ \to $$\Delta {H^ \circ }$
(B) A$ \to $energy of reactant, C$ \to $energy of product
(C)A$ \to $$\Delta {H^ \circ }$, C$ \to $activation energy
(D)A$ \to $Activation energy, C$ \to $threshold energy

Answer
566.7k+ views
Hint: The concept of threshold energy, activation energy, energy barrier, equilibrium and entropy are important to know in order to solve this question. This graph is the best representation of explaining all the above mentioned concepts of the distribution of potential energy and made learning easy.
Complete answer:
There is a small connection between the concept behind this question and collision theory. One of the postulates of collision theory states that “only those collisions results in a chemical reaction which is the colliding molecules are associated with a minimum energy called threshold energy” it means there is a minimum amount of energy the reacting molecules must acquire before reacting.
The activation energy that the excess energy a reactant molecule having less energy than, the threshold energy must acquire in order to react and form product. In words, we can define activation energy as the difference between the threshold energy and energy actually possessed by molecules. "A" in the graph depicts activation energy.
Enthalpy can be total energy stored in a system and its quantity depends upon the initial and final point and not on the path.
Let the initial state be ${H_a}$ and the final state be ${H_b}$. The change in enthalpy is given by:
\[\Delta H = {H_b} - {H_a}\]
Where, $\Delta H$ = Change in enthalpy
${H_a}$ = enthalpy at initial state
${H_b}$= enthalpy at final state
If, $\Delta H$ is positive then the reaction is endothermic and if $\Delta H$ is negative then the reaction is exothermic.
From the graph we can say, C is the difference between enthalpy of product and reactant which will be a positive quantity.
Thus, option A is the correct answer.
Additional information:
There is an energy barrier placed between the reactant and the product. In the above picture the point where the threshold energy and the point B inclines is the energy barrier according to the above picture. The barrier decides the magnitude of threshold energy. All reactants should cross this energy barrier in order to yield the product.
Note:
Enthalpy is nothing but the total heat content of the system. The energy contained per mole in the reactant and same goes to the product too. $\Delta H$ presents an endothermic reaction that is the heat absorbed during the reaction.
Complete answer:
There is a small connection between the concept behind this question and collision theory. One of the postulates of collision theory states that “only those collisions results in a chemical reaction which is the colliding molecules are associated with a minimum energy called threshold energy” it means there is a minimum amount of energy the reacting molecules must acquire before reacting.
The activation energy that the excess energy a reactant molecule having less energy than, the threshold energy must acquire in order to react and form product. In words, we can define activation energy as the difference between the threshold energy and energy actually possessed by molecules. "A" in the graph depicts activation energy.
Enthalpy can be total energy stored in a system and its quantity depends upon the initial and final point and not on the path.
Let the initial state be ${H_a}$ and the final state be ${H_b}$. The change in enthalpy is given by:
\[\Delta H = {H_b} - {H_a}\]
Where, $\Delta H$ = Change in enthalpy
${H_a}$ = enthalpy at initial state
${H_b}$= enthalpy at final state
If, $\Delta H$ is positive then the reaction is endothermic and if $\Delta H$ is negative then the reaction is exothermic.
From the graph we can say, C is the difference between enthalpy of product and reactant which will be a positive quantity.
Thus, option A is the correct answer.
Additional information:
There is an energy barrier placed between the reactant and the product. In the above picture the point where the threshold energy and the point B inclines is the energy barrier according to the above picture. The barrier decides the magnitude of threshold energy. All reactants should cross this energy barrier in order to yield the product.
Note:
Enthalpy is nothing but the total heat content of the system. The energy contained per mole in the reactant and same goes to the product too. $\Delta H$ presents an endothermic reaction that is the heat absorbed during the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




