
The plots of log V vs. log T at constant P for 1 mole of an ideal gas gives intercept equal to:
A.\[\log \dfrac{P}{R}\]
B.\[ - \dfrac{P}{R}\]
C.\[ - \dfrac{R}{P}\]
D.\[\log \dfrac{R}{P}\]
Answer
508.5k+ views
Hint: We can use the ideal gas equation to solve this problem. The ideal gas equation can be rearranged to obtain the graph. We know that the log V vs log T graph will yield us a straight line. By using the straight-line equation, we can say that y=mx+c and thus the value of c will give us the value of the intercept.
Complete answer:
According to the ideal gas equation we can say that
\[ \Rightarrow PV = nRT\]
This means that the pressure, volume, Temperature and the universal gas constant R can be related by this formula.
According to the question, we are given 1 mole of the gas. This means that the value of n=1.
Thus the equation becomes:
\[ \Rightarrow PV = RT\]
Applying logarithm on both the sides we get,
\[ \Rightarrow \log P + \log V = \log R + \log T\]
\[ \Rightarrow \log V = \log R + \log T - \log P\]
By simplifying the logarithm we will obtain:
\[ \Rightarrow \log V = \log \dfrac{R}{P} + \log T\]
This is of the form y=mx+c
Here log V is in the y-axis and log T is in the x-axis. Thus we can say that the value of c will be \[\log \dfrac{R}{P}\] .
The graph of the equation will be given by:
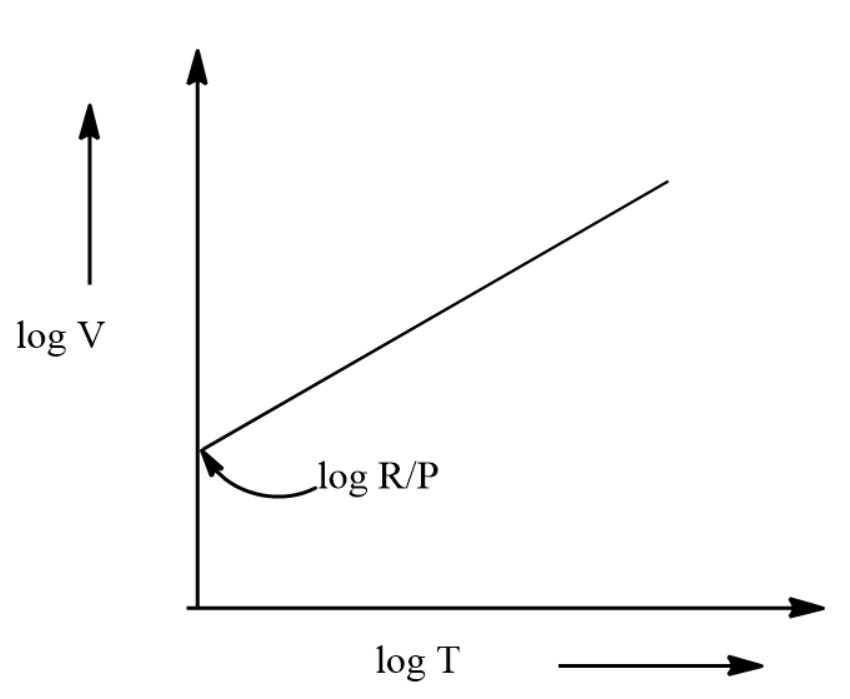
Hence the correct answer is option D.
Note:
While doing the simplification of the log terms we are taking the log P term to the right-hand side instead of log V because the value of P is a constant and thus the value of intercept obtained can be a constant. It is mentioned in the question that the experiment is done under constant pressure. Thus we need the intercept term to be a constant value and hence use P in it.
Complete answer:
According to the ideal gas equation we can say that
\[ \Rightarrow PV = nRT\]
This means that the pressure, volume, Temperature and the universal gas constant R can be related by this formula.
According to the question, we are given 1 mole of the gas. This means that the value of n=1.
Thus the equation becomes:
\[ \Rightarrow PV = RT\]
Applying logarithm on both the sides we get,
\[ \Rightarrow \log P + \log V = \log R + \log T\]
\[ \Rightarrow \log V = \log R + \log T - \log P\]
By simplifying the logarithm we will obtain:
\[ \Rightarrow \log V = \log \dfrac{R}{P} + \log T\]
This is of the form y=mx+c
Here log V is in the y-axis and log T is in the x-axis. Thus we can say that the value of c will be \[\log \dfrac{R}{P}\] .
The graph of the equation will be given by:

Hence the correct answer is option D.
Note:
While doing the simplification of the log terms we are taking the log P term to the right-hand side instead of log V because the value of P is a constant and thus the value of intercept obtained can be a constant. It is mentioned in the question that the experiment is done under constant pressure. Thus we need the intercept term to be a constant value and hence use P in it.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




