
The $ {\text{Phenol}}\xrightarrow{{{\text{Zn dust}}}}{\text{X}}\xrightarrow[{{\text{anhd}}{\text{. AlC}}{{\text{l}}_3}}]{{{\text{C}}{{\text{H}}_3}{\text{Cl}}}}{\text{Y}}\xrightarrow[{{\text{KMn}}{{\text{O}}_4}}]{{{\text{alkaline}}}}{\text{Z}} $ X
Answer
548.1k+ views
Hint: Zn dust plays a role of reducing agent. Here $ AlC{l_3} $ acts as a Lewis acid. Alkaline potassium permanganate acts as an oxidizing agent.
Complete Step by step solution:
Phenol reacts with zinc dust and undergoes reduction to form benzene. This is one of the important processes of preparation of benzene. Phenol first gets converted into phenoxide ion and proton gets released. The proton released will accept an electron from the zinc forming a H radical. As an effect of heating, there is homolytic fission which takes place in the carbon of the phenyl ring and $ {O^ - } $ .
The $ {O^ - } $ thus forms an oxide ion. Thus, zinc forms the zinc oxide and the phenyl radical which is produced, forms a bond with H radical. Benzene is formed.
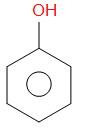 + Zn
+ Zn
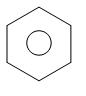
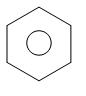 + ZnO
+ ZnO
phenol zinc benzene zinc oxide
The reaction of benzene with anhydrous $ AlC{l_3} $ and $ C{H_3}Cl $ is called Friedel-Crafts reaction. Friedel-Crafts reaction proceeds by an electrophilic aromatic substitution.
This reaction proceeds through carbocation rearrangement mechanisms.
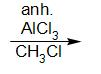
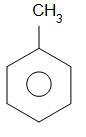
benzene toluene
The product formed is known as toluene.
Alkaline $ KMn{O_4} $ oxidises methyl group that is $ - C{H_3} $ group to the carboxylic acid group which is -COOH group. Hence benzoic acid is obtained as a product.
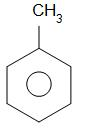
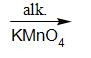
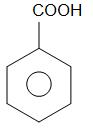
toluene benzoic acid
Therefore, the reaction of phenol with zinc dust gives product X as benzene ring, the reaction of benzene ring with anhydrous aluminium chloride and methyl chloride gives product Y as toluene and the reaction of toluene with alkaline potassium permanganate gives product Z as benzoic acid.
Note:
Benzoic acid that is formed as a product is an aromatic carboxylic acid. Alkaline $ KMn{O_4} $ has a great oxidising power as it provides nascent oxygen. Reducing agents are the reactants which themselves get oxidized and provide hydrogen to other reactants and oxidising agents are the reactants which itself get reduced and provide oxygen to other reactants.
Complete Step by step solution:
Phenol reacts with zinc dust and undergoes reduction to form benzene. This is one of the important processes of preparation of benzene. Phenol first gets converted into phenoxide ion and proton gets released. The proton released will accept an electron from the zinc forming a H radical. As an effect of heating, there is homolytic fission which takes place in the carbon of the phenyl ring and $ {O^ - } $ .
The $ {O^ - } $ thus forms an oxide ion. Thus, zinc forms the zinc oxide and the phenyl radical which is produced, forms a bond with H radical. Benzene is formed.



phenol zinc benzene zinc oxide
The reaction of benzene with anhydrous $ AlC{l_3} $ and $ C{H_3}Cl $ is called Friedel-Crafts reaction. Friedel-Crafts reaction proceeds by an electrophilic aromatic substitution.
This reaction proceeds through carbocation rearrangement mechanisms.

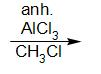

benzene toluene
The product formed is known as toluene.
Alkaline $ KMn{O_4} $ oxidises methyl group that is $ - C{H_3} $ group to the carboxylic acid group which is -COOH group. Hence benzoic acid is obtained as a product.

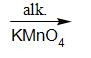

toluene benzoic acid
Therefore, the reaction of phenol with zinc dust gives product X as benzene ring, the reaction of benzene ring with anhydrous aluminium chloride and methyl chloride gives product Y as toluene and the reaction of toluene with alkaline potassium permanganate gives product Z as benzoic acid.
Note:
Benzoic acid that is formed as a product is an aromatic carboxylic acid. Alkaline $ KMn{O_4} $ has a great oxidising power as it provides nascent oxygen. Reducing agents are the reactants which themselves get oxidized and provide hydrogen to other reactants and oxidising agents are the reactants which itself get reduced and provide oxygen to other reactants.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




