
The pH of a carbonated drink is ___________.
(A) less than 7
(B) more than 7
(C) equal to 7
(D) approximately $ 7.8 $
Answer
556.2k+ views
Hint: pH is a scale used to identify acidity or basicity of an aqueous solution. Acidic solutions have lower pH value and basic or alkaline solutions have a higher pH value.
Complete Step by step solution:
The pH can be less than zero for very strong acids and greater than fourteen for very strong bases. At $ {25^ \circ } $ C, solutions with pH less than 7 are acidic and solutions with greater than 7 pH are basic. More precisely pH is negative of base 10 logarithm of hydrogen ion that is $ {H^ + } $ concentration. The concept of pH was introduced by Danish chemist Soren Dedec in the year 1909.
In carbonated drinks, there is a presence of carbon dioxide and water. Carbon dioxide reacts with water as the following reaction,
$ C{O_2} + {H_2}O \to {H_2}C{O_3} $
where $ C{O_2} $ is carbon dioxide, $ {H_2}O $ is water and $ {H_2}C{O_3} $ is carbonic acid.
Carbonic acid formed here is an acid and it releases $ {H^ + } $ ion and forms bicarbonate ion.
$ {H_2}C{O_3} \to {H^ + } + HC{O_3}^ - $
As carbonic acid releases $ {H^ + } $ ion, pH of carbonic acid decreases. And it becomes less than 7 due to an increase in concentration of $ {H^ + } $ ion. The pH of carbonated drinks is around 3 to 4.
Note:
In ocean acidification, carbonic acid is an important component. Its structure is
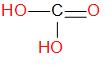
Carbonic acid plays an important role in the bicarbonate buffer system and it is used to maintain acid base homeostasis. Carbonic acid is also widely used in production of soft drinks and other bubbly beverages.
Complete Step by step solution:
The pH can be less than zero for very strong acids and greater than fourteen for very strong bases. At $ {25^ \circ } $ C, solutions with pH less than 7 are acidic and solutions with greater than 7 pH are basic. More precisely pH is negative of base 10 logarithm of hydrogen ion that is $ {H^ + } $ concentration. The concept of pH was introduced by Danish chemist Soren Dedec in the year 1909.
In carbonated drinks, there is a presence of carbon dioxide and water. Carbon dioxide reacts with water as the following reaction,
$ C{O_2} + {H_2}O \to {H_2}C{O_3} $
where $ C{O_2} $ is carbon dioxide, $ {H_2}O $ is water and $ {H_2}C{O_3} $ is carbonic acid.
Carbonic acid formed here is an acid and it releases $ {H^ + } $ ion and forms bicarbonate ion.
$ {H_2}C{O_3} \to {H^ + } + HC{O_3}^ - $
As carbonic acid releases $ {H^ + } $ ion, pH of carbonic acid decreases. And it becomes less than 7 due to an increase in concentration of $ {H^ + } $ ion. The pH of carbonated drinks is around 3 to 4.
Note:
In ocean acidification, carbonic acid is an important component. Its structure is

Carbonic acid plays an important role in the bicarbonate buffer system and it is used to maintain acid base homeostasis. Carbonic acid is also widely used in production of soft drinks and other bubbly beverages.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




