
The pair of metamers is:
(A)
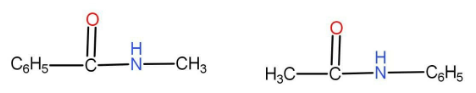
(B)
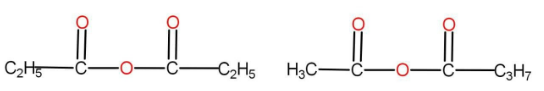
(C) Both the above
(D) None of the above.


Answer
555k+ views
Hint: To check whether the compounds given are metamers or not we just need to look at the alkyl group. If a different alkyl group is attached on either side then the compounds will be metamers.
Complete step by step solution:
The metamers are the structural isomers which occur due to the difference in the alkyl group attached at the same functional group in either side of the functional group.
The conditions for the formation of metamers are:
The molecular formula of the compounds given must be the same.
The type of alkyl group must be different.
The functional group must be the same in the compounds.
The option A given is
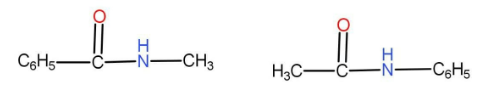
We can see that both of them have the same numbers of atoms. The functional group is carbonyl derivative. As we can see that there are different alkyl groups present on either side of the functional group. In one of them the methyl group is on the right hand side and in the second one the methyl group is on the left hand side. Hence these two compounds are metamers.
The option B is
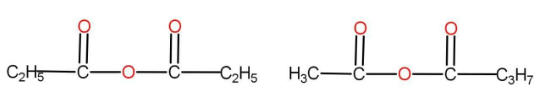
There are the same numbers of atoms present in both of them. The functional group here is anhydride. There are different alkyl groups present in both the compounds. Hence, they are also metamers.
Both the option A and option B are metamers. Hence, the correct option is C.
Note:
Isomers are those compounds which have the same molecular formula but different structural or special arrangements. There are two types of isomers: structural isomers and stereoisomers. Structural isomers are further of many types such as functional isomers, position isomers, tautomers, metamers, linkage isomers etc.
Complete step by step solution:
The metamers are the structural isomers which occur due to the difference in the alkyl group attached at the same functional group in either side of the functional group.
The conditions for the formation of metamers are:
The molecular formula of the compounds given must be the same.
The type of alkyl group must be different.
The functional group must be the same in the compounds.
The option A given is

We can see that both of them have the same numbers of atoms. The functional group is carbonyl derivative. As we can see that there are different alkyl groups present on either side of the functional group. In one of them the methyl group is on the right hand side and in the second one the methyl group is on the left hand side. Hence these two compounds are metamers.
The option B is

There are the same numbers of atoms present in both of them. The functional group here is anhydride. There are different alkyl groups present in both the compounds. Hence, they are also metamers.
Both the option A and option B are metamers. Hence, the correct option is C.
Note:
Isomers are those compounds which have the same molecular formula but different structural or special arrangements. There are two types of isomers: structural isomers and stereoisomers. Structural isomers are further of many types such as functional isomers, position isomers, tautomers, metamers, linkage isomers etc.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




