
The oxidation number of phosphorus in $PO_{4}^{3-}$, ${{P}_{4}}{{O}_{10}}$ and ${{P}_{2}}O_{7}^{4-}$ is:
(A)- +5
(B)- +3
(C)- -3
(D)- +2
Answer
598.8k+ views
Hint: Oxidation number is the charge obtained by the atom of an element when it loses or gains electrons while going from its free state to combined state with atoms of other elements. It can be positive, negative or zero.
Oxidation number of oxygen is -2 in all of its compounds with a few exceptions like peroxides, superoxides, etc.
Complete step by step answer:
Let us calculate the oxidation number of phosphorus in $PO_{4}^{3-}$, ${{P}_{4}}{{O}_{10}}$ and ${{P}_{2}}O_{7}^{4-}$ one by one.
Oxidation number of P in phosphate anion $PO_{4}^{3-}$ is calculated as follows:
Structure of $PO_{4}^{3-}$

One unit negative charge is equal to two electrons in a bond. It is clear from the structure that all the oxygen atoms are in -2 oxidation state.
Let the oxidation number of P be ‘$x$’.
Oxidation number of O is -2.
\[PO_{4}^{3-}\] is a negatively charged ion. The total charge on the ion is -3.
Now, we can write
\[\begin{align}
& x+4(-2)=-3 \\
& x-8=-3 \\
& x=8-3=5 \\
\end{align}\]
Oxidation number of P in phosphorus pentoxide ${{P}_{4}}{{O}_{10}}$:
Structure of ${{P}_{4}}{{O}_{10}}$
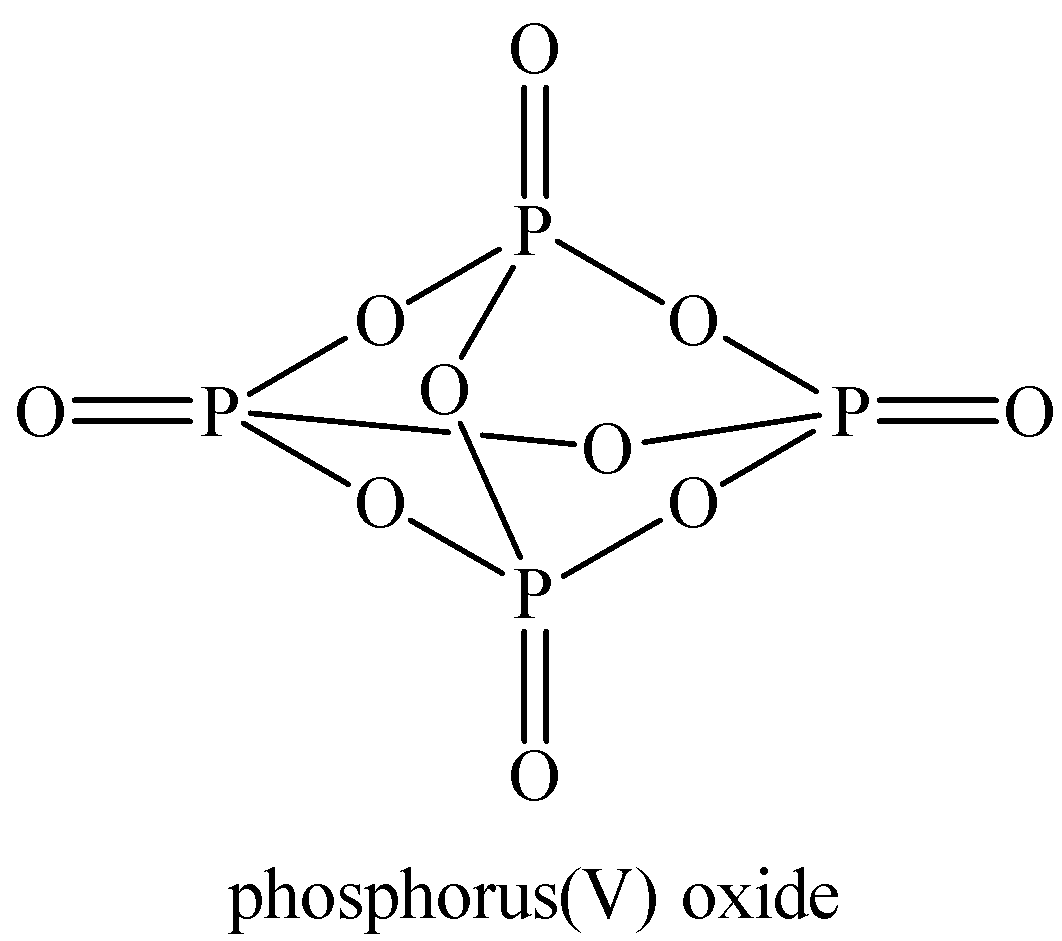
Now, we can see all the P atoms are in the same electronic environment and thus, have the same oxidation number.
Let the oxidation number of P be ‘$x$’.
Oxidation of O atoms is -2.
${{P}_{4}}{{H}_{10}}$ is a neutral compound, so the total charge on it is taken as zero.
Now we can calculate the oxidation number of P in ${{P}_{4}}{{O}_{10}}$ as
\[\begin{align}
& 4(x)+10(-2)=0 \\
& 4x=20\Rightarrow x=+5 \\
\end{align}\]
Oxidation number of P in pyrophosphate ion ${{P}_{2}}O_{7}^{4-}$ :
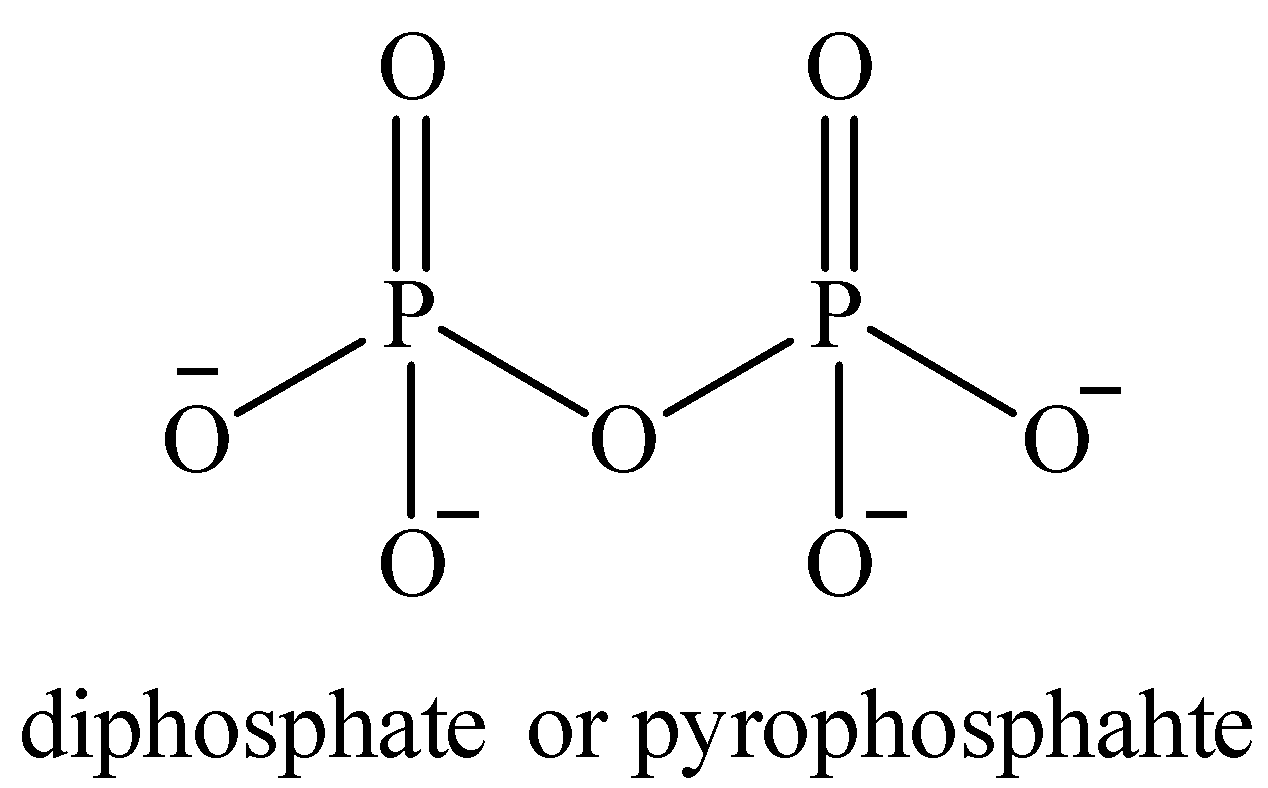
Structure of ${{P}_{2}}O_{7}^{4-}$
Now, the oxidation number of both the P atoms is the same. Let it be ‘$x$’.
All the O atoms are in -2 oxidation state.
Total charge on the compound is -4. So the oxidation number of P is calculated as follows:
\[\begin{align}
& 2(x)+7(-2)=-4 \\
& 2x-14=-4 \\
& 2x=10\Rightarrow x=+5 \\
\end{align}\]
\[\]
Therefore, the oxidation number of phosphorus in $PO_{4}^{3-}$, ${{P}_{4}}{{O}_{10}}$ and ${{P}_{2}}O_{7}^{4-}$ is +5.
So, the correct answer is “Option A”.
Note: Phosphorous is a group 15 element. It has 5 electrons in the valence shell. Oxidation number of an element in a combined state is affected by the bonding behavior. Oxidation state of phosphorus varies from – 3 in compounds with metals to +5 with electronegative elements.
Oxidation number of oxygen is -2 in all of its compounds with a few exceptions like peroxides, superoxides, etc.
Complete step by step answer:
Let us calculate the oxidation number of phosphorus in $PO_{4}^{3-}$, ${{P}_{4}}{{O}_{10}}$ and ${{P}_{2}}O_{7}^{4-}$ one by one.
Oxidation number of P in phosphate anion $PO_{4}^{3-}$ is calculated as follows:
Structure of $PO_{4}^{3-}$

One unit negative charge is equal to two electrons in a bond. It is clear from the structure that all the oxygen atoms are in -2 oxidation state.
Let the oxidation number of P be ‘$x$’.
Oxidation number of O is -2.
\[PO_{4}^{3-}\] is a negatively charged ion. The total charge on the ion is -3.
Now, we can write
\[\begin{align}
& x+4(-2)=-3 \\
& x-8=-3 \\
& x=8-3=5 \\
\end{align}\]
Oxidation number of P in phosphorus pentoxide ${{P}_{4}}{{O}_{10}}$:
Structure of ${{P}_{4}}{{O}_{10}}$

Now, we can see all the P atoms are in the same electronic environment and thus, have the same oxidation number.
Let the oxidation number of P be ‘$x$’.
Oxidation of O atoms is -2.
${{P}_{4}}{{H}_{10}}$ is a neutral compound, so the total charge on it is taken as zero.
Now we can calculate the oxidation number of P in ${{P}_{4}}{{O}_{10}}$ as
\[\begin{align}
& 4(x)+10(-2)=0 \\
& 4x=20\Rightarrow x=+5 \\
\end{align}\]
Oxidation number of P in pyrophosphate ion ${{P}_{2}}O_{7}^{4-}$ :

Structure of ${{P}_{2}}O_{7}^{4-}$
Now, the oxidation number of both the P atoms is the same. Let it be ‘$x$’.
All the O atoms are in -2 oxidation state.
Total charge on the compound is -4. So the oxidation number of P is calculated as follows:
\[\begin{align}
& 2(x)+7(-2)=-4 \\
& 2x-14=-4 \\
& 2x=10\Rightarrow x=+5 \\
\end{align}\]
\[\]
Therefore, the oxidation number of phosphorus in $PO_{4}^{3-}$, ${{P}_{4}}{{O}_{10}}$ and ${{P}_{2}}O_{7}^{4-}$ is +5.
So, the correct answer is “Option A”.
Note: Phosphorous is a group 15 element. It has 5 electrons in the valence shell. Oxidation number of an element in a combined state is affected by the bonding behavior. Oxidation state of phosphorus varies from – 3 in compounds with metals to +5 with electronegative elements.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




