
The order of stability of the following carbocation:
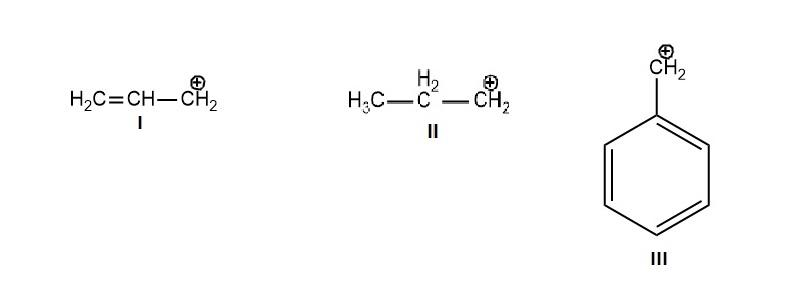
A- II > III > I
B- I > II > III
C- III > I > II
D- III > II > I

Answer
578.7k+ views
Hint: Decide the stability of given carbocation by the number of resonating structures they can form. More resonating structures represents more stable carbocation.
Complete step by step solution:
Resonance stabilizes the structure and lowers the energy of the carbocation, as the question is asking about the stability of given carbocation, we will check first which structure shows more resonating structure. More number of resonating structure means more stable the cation is. Hyperconjugation also stabilizes the carbocation but its effect on stability is less considerable then that of by resonance.
-In, I structure there are 2 resonating structures of the compound.
-In, II structure there is no resonance but in this structure hyperconjugation is present (which can form 2 conjugating structures) which stabilize it.
-In III structure there are 5 resonating structures of the compound.
-In this way structure I has 2 and III has 5 resonating structures and structure II has 2 conjugating structures. From these numbers of resonating or conjugating structures, we can predict the stability order of them. Stability order of the given 3 compounds is III > I > II
Hence, the correct option is (C).
Note: Resonance provides more stability than that of hyperconjugation. That’s why among structure I and II, I is more stable, even though they have 2 resonating and 2 conjugating structures respectively.
Complete step by step solution:
Resonance stabilizes the structure and lowers the energy of the carbocation, as the question is asking about the stability of given carbocation, we will check first which structure shows more resonating structure. More number of resonating structure means more stable the cation is. Hyperconjugation also stabilizes the carbocation but its effect on stability is less considerable then that of by resonance.
-In, I structure there are 2 resonating structures of the compound.
-In, II structure there is no resonance but in this structure hyperconjugation is present (which can form 2 conjugating structures) which stabilize it.
-In III structure there are 5 resonating structures of the compound.
-In this way structure I has 2 and III has 5 resonating structures and structure II has 2 conjugating structures. From these numbers of resonating or conjugating structures, we can predict the stability order of them. Stability order of the given 3 compounds is III > I > II
Hence, the correct option is (C).
Note: Resonance provides more stability than that of hyperconjugation. That’s why among structure I and II, I is more stable, even though they have 2 resonating and 2 conjugating structures respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




