
The number of vibrational degrees of freedom for a $C{{O}_{2}}$ molecule is
a) 4
b) 5
c) 6
d) 9
Answer
591.6k+ views
Hint: Though carbon dioxide is a triatomic molecule, it is not arranged in a triangular fashion. It is a linear molecule with all the three atoms arranged in the same line. Hence we would use the relation between the number of linkages of the atoms of the molecule to determine the vibrational degrees of freedom for a $C{{O}_{2}}$.
Complete step-by-step answer:
Let us first understand what does degrees of freedom in a molecule means,
Degrees of freedom of a system or a molecule is the number of independent ways in which the particles of the system can absorb energy.
It can also be defined as the number of coordinates required to specify the position of the constituent particles of the system minus the number of independent relation, i.e.
$f=3N-k$ where f is the degrees of freedom, N is the number of particles in the system and k is the independent relation between the particles.
The above relation that we obtained is for total degrees of freedom of a molecule at moderate temperatures. But at higher temperatures the molecule possesses an additional degree of freedom that is a vibrational degree of freedom. Hence, now the number of degrees of freedom of a molecule with N particles is given by,
$f=\text{Translational + Rotational + Vibrational}...(1)$. The total number of degrees of freedom for a linear molecule is given by 3N, where N is the no of atoms of the molecule. For a linear carbon dioxide molecule, it has 3 translational and 2 rotational degrees of freedom hence the equation 1 becomes,
$\begin{align}
& f=3+2+\text{Vibrational} \\
& \text{Vibrational}=3N-5 \\
\end{align}$
A $C{{O}_{2}}$molecule has 3 atoms, two of oxygen and one carbon atom. Hence the vibrational degrees of freedom of the molecule is,
$\begin{align}
& \text{Vibrational}=3N-5 \\
& \text{Vibrational}=3(3)-5 \\
& \text{Vibrational}=9-5=4 \\
\end{align}$
Hence the carbon dioxide molecule has 4 vibrational degrees of freedom. Hence the correct answer is option a.
Note:
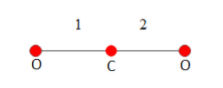
In a symmetric carbon dioxide molecule as shown above, the two oxygen molecules vibrate about the carbon molecule. Each vibrational motion has both kinetic and potential energies. So one degree of freedom of vibrational motion is taken as two. Since the above molecule vibrates along two linkages we can directly conclude it has four degrees of freedom.
Complete step-by-step answer:
Let us first understand what does degrees of freedom in a molecule means,
Degrees of freedom of a system or a molecule is the number of independent ways in which the particles of the system can absorb energy.
It can also be defined as the number of coordinates required to specify the position of the constituent particles of the system minus the number of independent relation, i.e.
$f=3N-k$ where f is the degrees of freedom, N is the number of particles in the system and k is the independent relation between the particles.
The above relation that we obtained is for total degrees of freedom of a molecule at moderate temperatures. But at higher temperatures the molecule possesses an additional degree of freedom that is a vibrational degree of freedom. Hence, now the number of degrees of freedom of a molecule with N particles is given by,
$f=\text{Translational + Rotational + Vibrational}...(1)$. The total number of degrees of freedom for a linear molecule is given by 3N, where N is the no of atoms of the molecule. For a linear carbon dioxide molecule, it has 3 translational and 2 rotational degrees of freedom hence the equation 1 becomes,
$\begin{align}
& f=3+2+\text{Vibrational} \\
& \text{Vibrational}=3N-5 \\
\end{align}$
A $C{{O}_{2}}$molecule has 3 atoms, two of oxygen and one carbon atom. Hence the vibrational degrees of freedom of the molecule is,
$\begin{align}
& \text{Vibrational}=3N-5 \\
& \text{Vibrational}=3(3)-5 \\
& \text{Vibrational}=9-5=4 \\
\end{align}$
Hence the carbon dioxide molecule has 4 vibrational degrees of freedom. Hence the correct answer is option a.
Note:

In a symmetric carbon dioxide molecule as shown above, the two oxygen molecules vibrate about the carbon molecule. Each vibrational motion has both kinetic and potential energies. So one degree of freedom of vibrational motion is taken as two. Since the above molecule vibrates along two linkages we can directly conclude it has four degrees of freedom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




