
The number of structural isomers possible for ${C_4}{H_8}$ is.
1) $4$
2) $3$
3) $5$
4) $6$
Answer
522.5k+ views
Hint: We know that the molecules with the same molecular formula but different molecular geometries are called isomers. There are a number of types of isomers. They are,
Conformational isomers.
Constitutional isomers.
Complete step by step answer:
We must know that an alkane with 4 carbon atoms would have the formula \[\;{C_4}{H_{10}}\] but the given hydrocarbon has two hydrogens less, so it must contain either a double bond or a ring. There are five possible isomers. They are But-1-ene, But-2-ene, 2-Methylpropane, Cyclobutane, and methyl cyclopropane.We can draw the all possible isomeric structures as,
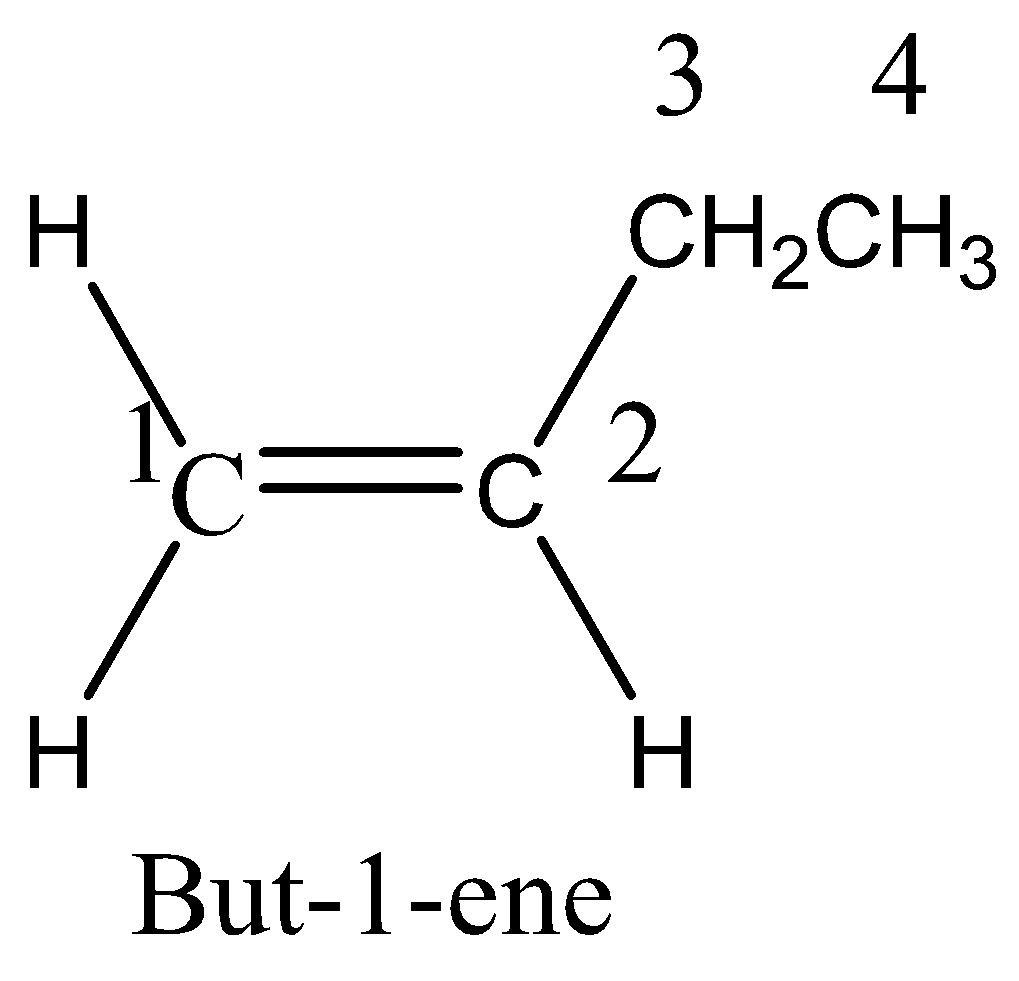
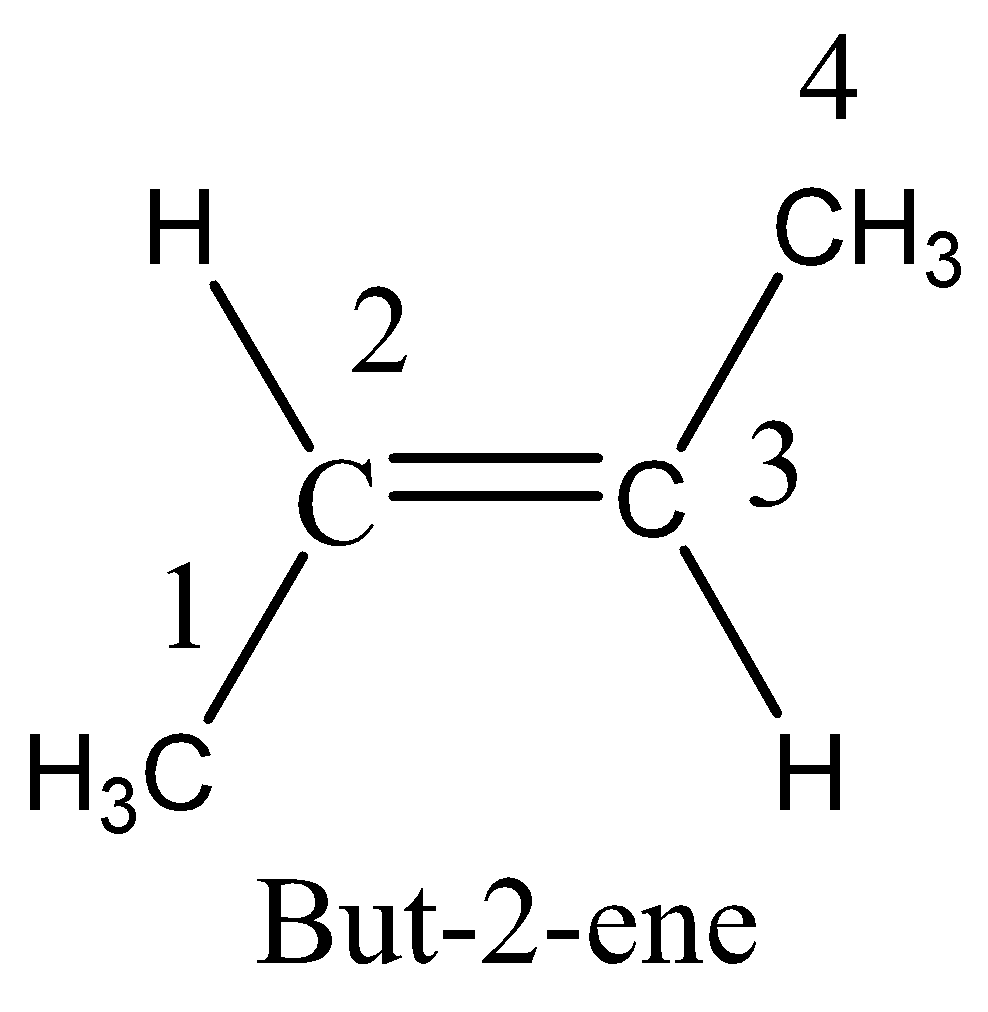
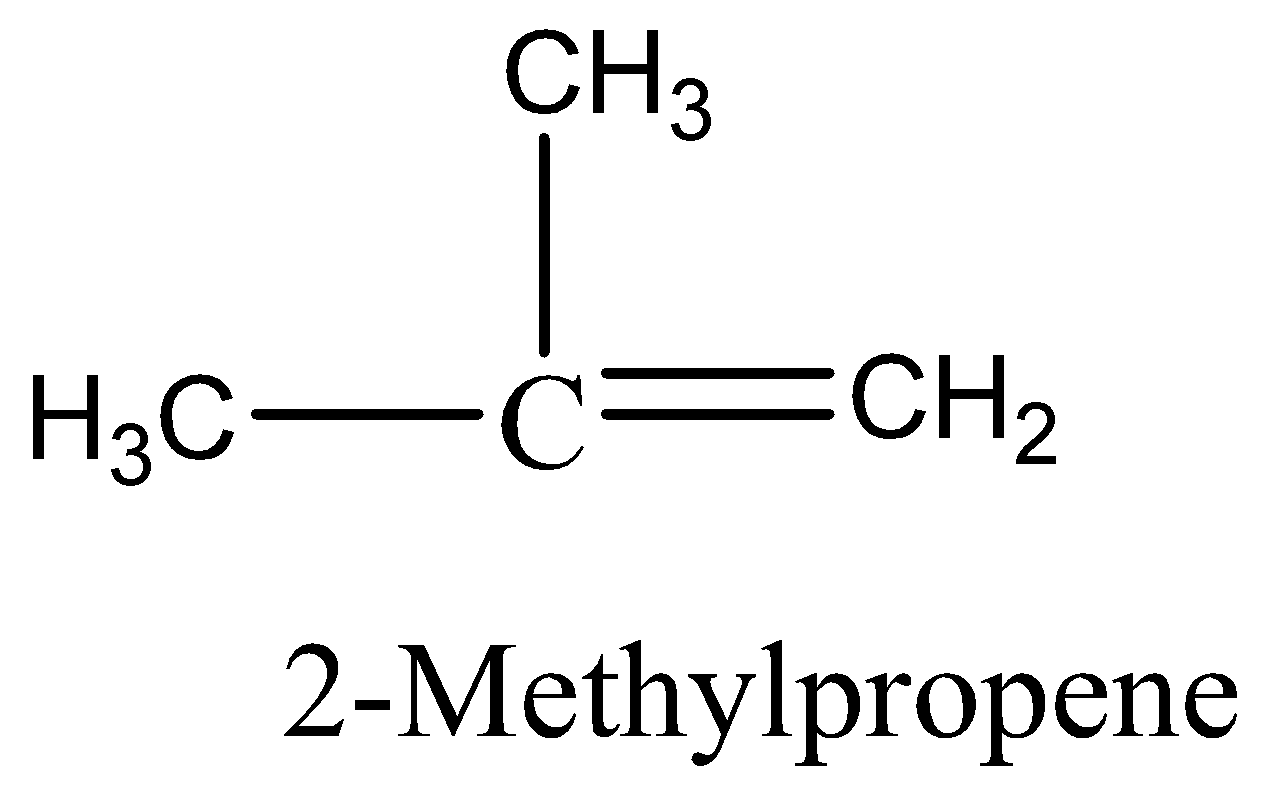
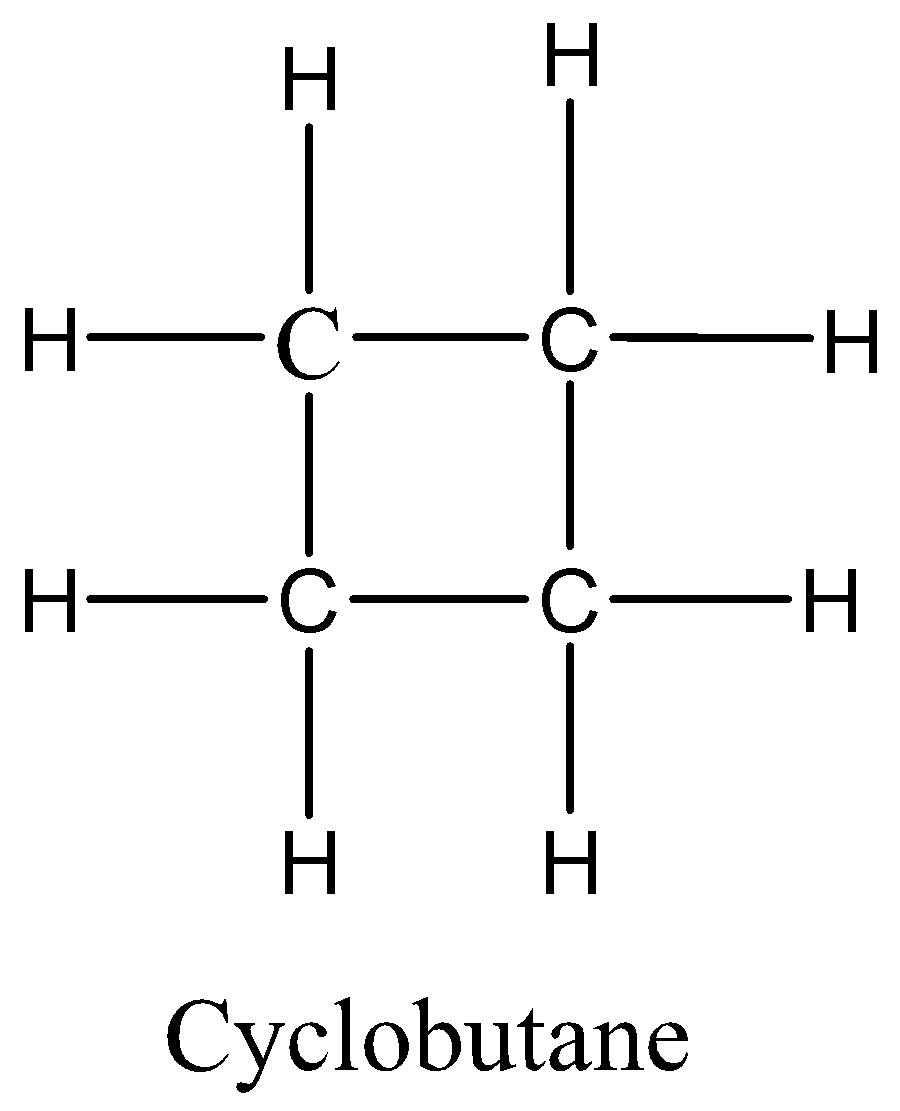
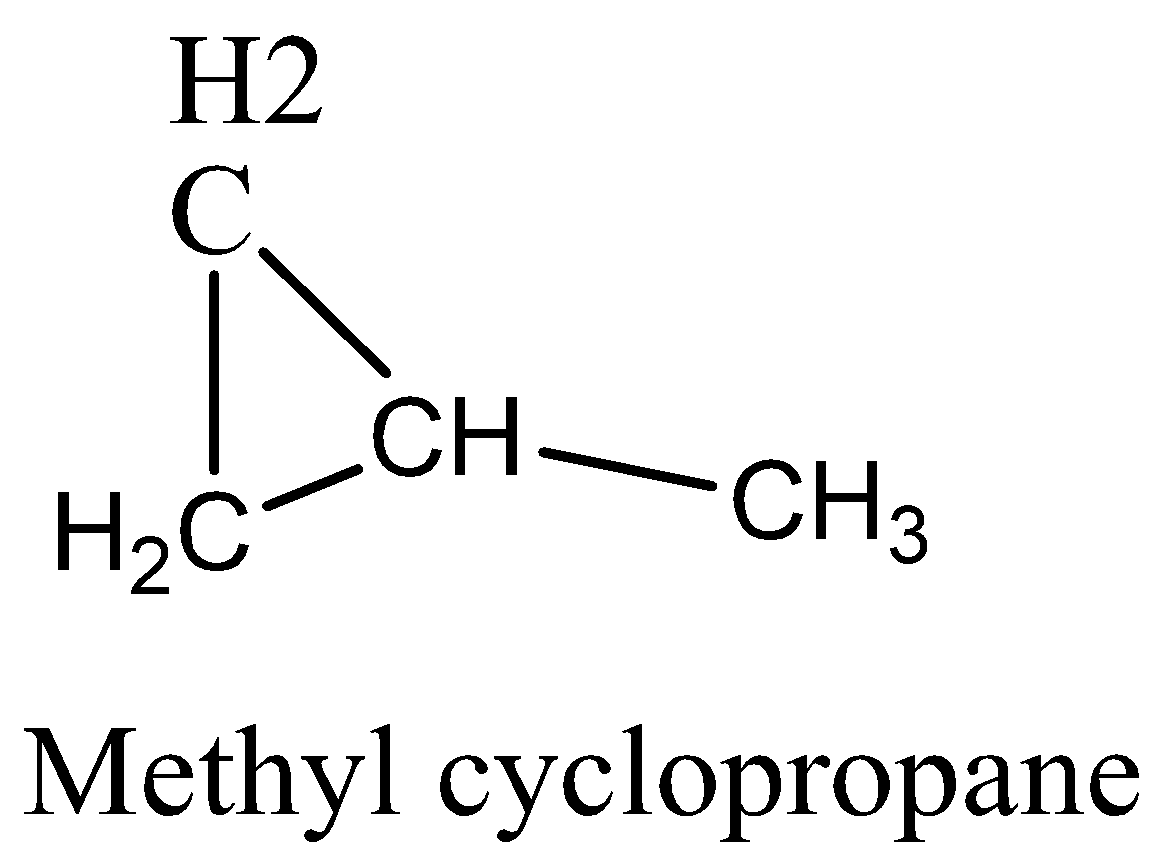
Therefore, the option 3 is correct.
Additional Information:
We know that if there is no superimposable mirror image and there is no plane of symmetry in the molecule then the molecule is called a chiral. This property is known as chirality and the compounds with the same molecular formula but differ in the arrangement of the atom are said to be isomers. The compounds which are mirror images but are not identical; to each other are called enantiomers.
Note:
Now we discuss how structural isomers, stereoisomers and conformational isomers differ from each other.
1.Structural isomer:
The molecules which have the same molecular formula but differ by the atoms or bonds are called a 2.structural isomer. It is also known as a constitutional isomer. There are two types of Structural isomer; they are (1) chain isomers and (2) ring isomers.
3.Stereoisomers:
The isomers which differ by the orientation of atoms in space are called stereoisomers. There are two types of Stereoisomers; they are (1) Geometrical isomer and (2) optical isomer.
Conformational isomer:
4.The isomers that are different by their rotation around a single bond are called Conformational isomers.
Conformational isomers.
Constitutional isomers.
Complete step by step answer:
We must know that an alkane with 4 carbon atoms would have the formula \[\;{C_4}{H_{10}}\] but the given hydrocarbon has two hydrogens less, so it must contain either a double bond or a ring. There are five possible isomers. They are But-1-ene, But-2-ene, 2-Methylpropane, Cyclobutane, and methyl cyclopropane.We can draw the all possible isomeric structures as,





Therefore, the option 3 is correct.
Additional Information:
We know that if there is no superimposable mirror image and there is no plane of symmetry in the molecule then the molecule is called a chiral. This property is known as chirality and the compounds with the same molecular formula but differ in the arrangement of the atom are said to be isomers. The compounds which are mirror images but are not identical; to each other are called enantiomers.
Note:
Now we discuss how structural isomers, stereoisomers and conformational isomers differ from each other.
1.Structural isomer:
The molecules which have the same molecular formula but differ by the atoms or bonds are called a 2.structural isomer. It is also known as a constitutional isomer. There are two types of Structural isomer; they are (1) chain isomers and (2) ring isomers.
3.Stereoisomers:
The isomers which differ by the orientation of atoms in space are called stereoisomers. There are two types of Stereoisomers; they are (1) Geometrical isomer and (2) optical isomer.
Conformational isomer:
4.The isomers that are different by their rotation around a single bond are called Conformational isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




