
The number of sigma and pi-bonds in 1-butene 3-yne are:
(A) 5 sigma and 5 pi
(B) 7 sigma and 3 pi
(C) 8 sigma and 2 pi
(D) 6 sigma and 4 pi
Answer
590.1k+ views
Hint: Pi Bonds can form in double and triple bonds but do not form in single bonds in most cases. Pi bonds are generally weaker than sigma bonds because they are formed of lateral overlapping, however, sigma bonds are formed by axial overlapping.
Complete step by step answer:
The structure of 1-butene 3-yne is as follows:
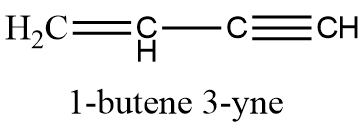
Sigma bonds ($\sigma $bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Single bonds are formed by sigma bonds. Axial overlapping of s-s orbitals, and s-p and p-p orbitals results in formation of sigma bonds.
Pi bonds ($\pi $ bonds) are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally.
The compound 1-butene 3-yne has 4 C-H single bonds, that is 4 sigma bonds, and 3 C-C sigma bonds, therefore, there are a total of 7 sigma bonds.
The compound has one double bond and one triple bond. Each double bond has one sigma and one pi bond , and each triple bond has one sigma and two pi bonds, therefore, the compound has 3 pi bonds. So, the correct answer is “Option B”.
Note: Each of these atomic orbitals in $\pi $ bonds has zero electron density at a shared nodal plane, passing through the two bonded nuclei. The same plane is also a nodal plane for the molecular orbital of the pi bond.
Complete step by step answer:
The structure of 1-butene 3-yne is as follows:
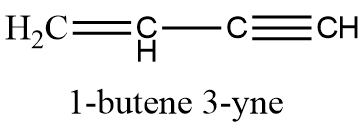
Sigma bonds ($\sigma $bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Single bonds are formed by sigma bonds. Axial overlapping of s-s orbitals, and s-p and p-p orbitals results in formation of sigma bonds.
Pi bonds ($\pi $ bonds) are covalent chemical bonds where two lobes of an orbital on one atom overlap two lobes of an orbital on another atom and this overlap occurs laterally.
The compound 1-butene 3-yne has 4 C-H single bonds, that is 4 sigma bonds, and 3 C-C sigma bonds, therefore, there are a total of 7 sigma bonds.
The compound has one double bond and one triple bond. Each double bond has one sigma and one pi bond , and each triple bond has one sigma and two pi bonds, therefore, the compound has 3 pi bonds. So, the correct answer is “Option B”.
Note: Each of these atomic orbitals in $\pi $ bonds has zero electron density at a shared nodal plane, passing through the two bonded nuclei. The same plane is also a nodal plane for the molecular orbital of the pi bond.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




