
The number of resonating structures of \[N{O_2}\] is:
A. 3
B. 2
C. 5
D. 4
Answer
570k+ views
Hint: We have to remember that the resonance structures are a group of Lewis structures which collectively describes the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. They are capable of describing the delocalized electrons that can’t be expressed by a single Lewis formula.
Complete step by step answer:
Let us discuss the structure of \[N{O_2}\]. The structure of \[N{O_2}\] can’t be explained by a single Lewis structure. The structure of \[N{O_2}\] is non-cyclic and represented as,
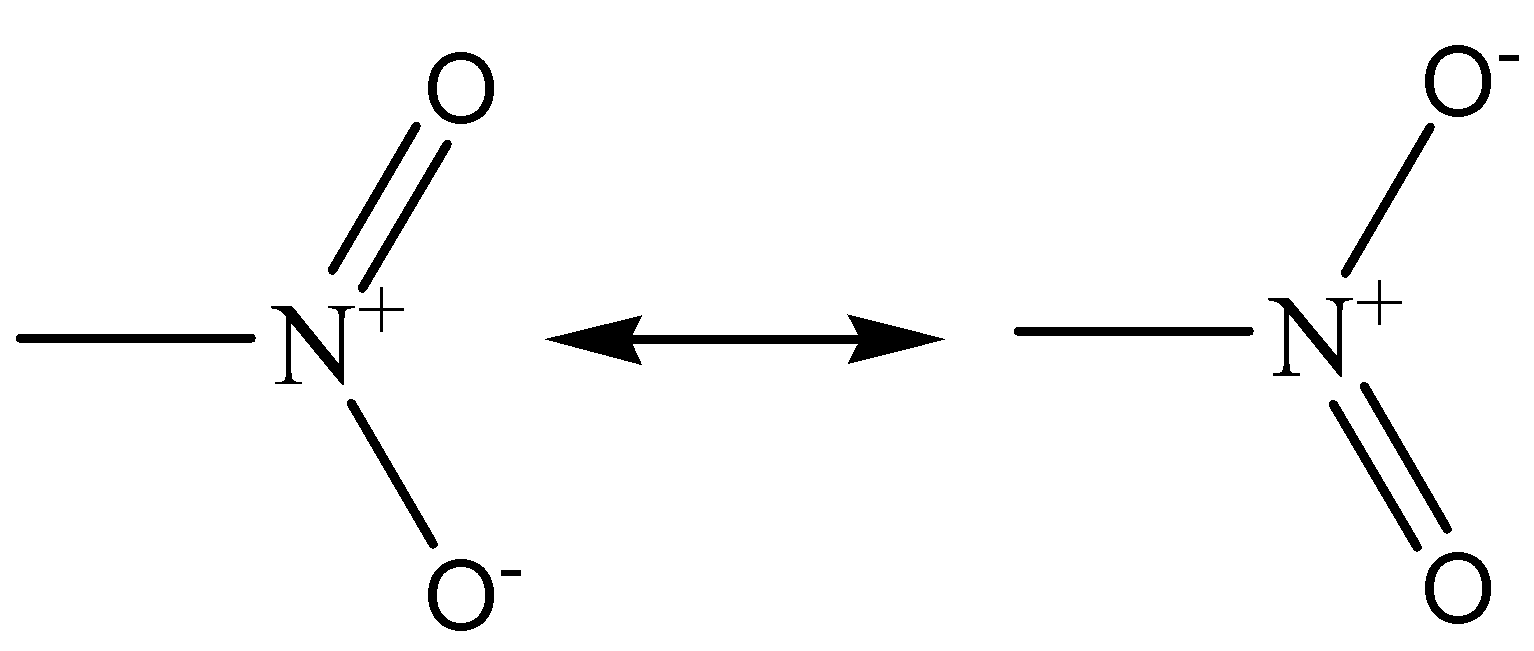
As per above representation each has an \[N = O\] double bond and an $N -{O^ {-}}$ bond. The N atom has an unpaired electron and a positive charge. These are the two major resonances that contribute to \[N{O_2}\].
So, the correct answer is Option B.
Note: We have to remember that the energy of resonance hybrid is lower than that of its resonating structure.
As we know that the difference in energy between hybrid structure and resonating structure is named as resonance energy.
The more the number of resonating structures the more is resonance energy.
Resonance structures are hypothetical and don’t represent any important molecule. The important structure of a molecule is a hybrid of the resonating structure.
Phenol and nitrobenzene are often explained by Lewis structure, because the structure of benzene is cyclic.
We can arrange order of activating groups as,
\[ - N{H_2} > N{R_2} > - OH, - OR > - NHCOR > - C{H_3}\] and other alkyl groups
We can give an order of deactivating group as,
\[ - N{O_2} > C{F_3} > - COR > - CN > - C{O_2}R > - S{O_3}H > {\text{halogens}}\]
Place the empty octet on the most substituted carbon (remember carbocation stability). Avoid placing positive charge adjacent to electron withdrawing groups if possible.
Complete step by step answer:
Let us discuss the structure of \[N{O_2}\]. The structure of \[N{O_2}\] can’t be explained by a single Lewis structure. The structure of \[N{O_2}\] is non-cyclic and represented as,

As per above representation each has an \[N = O\] double bond and an $N -{O^ {-}}$ bond. The N atom has an unpaired electron and a positive charge. These are the two major resonances that contribute to \[N{O_2}\].
So, the correct answer is Option B.
Note: We have to remember that the energy of resonance hybrid is lower than that of its resonating structure.
As we know that the difference in energy between hybrid structure and resonating structure is named as resonance energy.
The more the number of resonating structures the more is resonance energy.
Resonance structures are hypothetical and don’t represent any important molecule. The important structure of a molecule is a hybrid of the resonating structure.
Phenol and nitrobenzene are often explained by Lewis structure, because the structure of benzene is cyclic.
We can arrange order of activating groups as,
\[ - N{H_2} > N{R_2} > - OH, - OR > - NHCOR > - C{H_3}\] and other alkyl groups
We can give an order of deactivating group as,
\[ - N{O_2} > C{F_3} > - COR > - CN > - C{O_2}R > - S{O_3}H > {\text{halogens}}\]
Place the empty octet on the most substituted carbon (remember carbocation stability). Avoid placing positive charge adjacent to electron withdrawing groups if possible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




