
The number of possible resonance structures for $CO_3^{ - 2}$ is:
A. $2$
B. $3$
C. $6$
D. $9$
Answer
579k+ views
Hint: Lewis systems, furthermore mentioned to as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot systems, or Lewis electron dot structures (LEDS), are figures that show the bonding between atoms of a molecule and the lone pairs of electrons that could exist inside the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Complete step by step solution:
Unlike ${O_3}$, even though the actual shape of $CO_3^{ - 2}$ is an average of three resonance structures. $2$ Carbon has four valence electrons, every oxygen has $6$ valence electrons, and there are $2$ extra for the $ - 2$ price. This gives $4 + (3\times 6) + 2 = 24$ valence electrons.
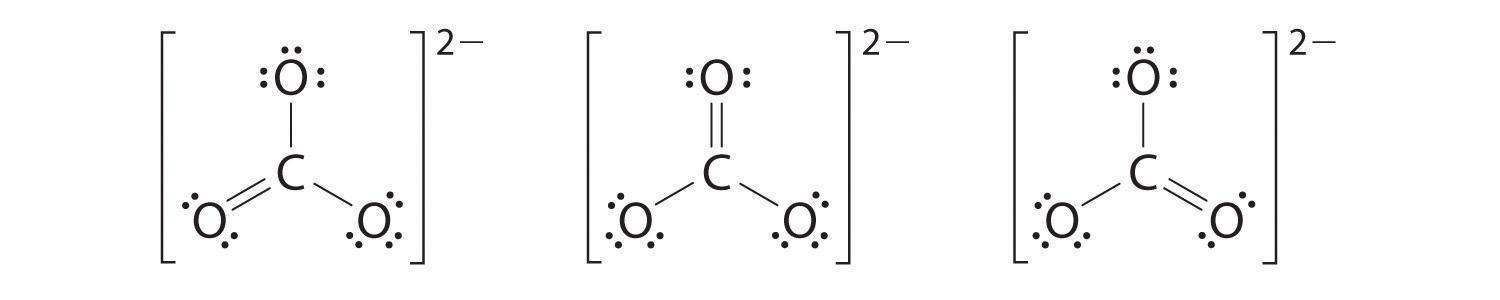
So, the correct answer is B.
Additional information:
For a few molecules and ions, it's tough to determine which lone pairs must be moved to form double or triple bonds, and or extra exclusive resonance systems can be written for the identical molecule or ion. In such cases it is typical to put in writing they all with -manner arrows in between (see Example under). This is occasionally the case while more than one atom of the equal type surrounds the imperative atom, and is specifically common for polyatomic ions.
When this situation happens, the molecule's Lewis shape is stated to be a resonance structure, and the molecule exists as a resonance hybrid. Each of the distinct possibilities is superimposed on the others, and the molecule is taken into consideration to have a Lewis structure equivalent to some mixture of these states.
Note: The formal charge of an atom is computed because the difference among the quantity of valence electrons that a neutral atom might have and the quantity of electrons that belong to it in the Lewis structure. Electrons in covalent bonds break up equally between the atoms worried inside the bond. The overall of the formal prices on an ion need to be the same as the fee at the ion, and the full of the formal fees on an impartial molecule have to be the same to zero.
Complete step by step solution:
Unlike ${O_3}$, even though the actual shape of $CO_3^{ - 2}$ is an average of three resonance structures. $2$ Carbon has four valence electrons, every oxygen has $6$ valence electrons, and there are $2$ extra for the $ - 2$ price. This gives $4 + (3\times 6) + 2 = 24$ valence electrons.

So, the correct answer is B.
Additional information:
For a few molecules and ions, it's tough to determine which lone pairs must be moved to form double or triple bonds, and or extra exclusive resonance systems can be written for the identical molecule or ion. In such cases it is typical to put in writing they all with -manner arrows in between (see Example under). This is occasionally the case while more than one atom of the equal type surrounds the imperative atom, and is specifically common for polyatomic ions.
When this situation happens, the molecule's Lewis shape is stated to be a resonance structure, and the molecule exists as a resonance hybrid. Each of the distinct possibilities is superimposed on the others, and the molecule is taken into consideration to have a Lewis structure equivalent to some mixture of these states.
Note: The formal charge of an atom is computed because the difference among the quantity of valence electrons that a neutral atom might have and the quantity of electrons that belong to it in the Lewis structure. Electrons in covalent bonds break up equally between the atoms worried inside the bond. The overall of the formal prices on an ion need to be the same as the fee at the ion, and the full of the formal fees on an impartial molecule have to be the same to zero.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




