
The number of ${\text{P - O}}$ bonds in ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$ is?
A) 9
B) 6
C) 12
D) 18
Answer
548.4k+ views
Hint:
To answer this question you must recall the oxides of phosphorus and their structures. ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$ is a dimer of ${{\text{P}}_2}{{\text{O}}_3}$.
Complete step by step solution:
${{\text{P}}_2}{{\text{O}}_3}$/ ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$ (phosphorus trioxide) is also known as phosphorous oxide or phosphorous anhydride because it is the anhydride of orthophosphoric acid. It is a soft white solid.
We know that since it is anhydride of an acid it will be acidic itself also and hydrolysis in water to form phosphorous acid. Phosphorus trioxide exists as a dimer and is written as ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$.
The structure of phosphorus trioxide dimer is,
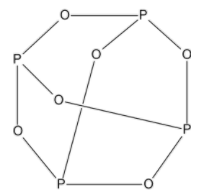
From the structure, we can clearly see that each phosphorus atom is bonded to three oxygen atoms in a cyclic structure.
Thus, there are 12 ${\text{P - O}}$ bonds in ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$.
The correct option is C.
Note:
Phosphorus forms two common oxides one of which is phosphorus trioxide.
The other common oxide of phosphorus is phosphorus pentoxide.
Phosphorus pentoxide has a molecular formula ${{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}$ and its common name is derived from its empirical formula,${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}$. It is a white crystalline solid. It is the anhydride of ortho phosphoric acid. It is a very powerful and useful desiccant and dehydrating agent.
Phosphorus pentoxide majorly crystallizes in four forms which are also known as polymorphs. The most common one is a metastable form, comprises molecules of ${{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}$. These molecules are held together in a hexagonal lattice. This form can be prepared by condensing the vapour of phosphorus pentoxide rapidly, resulting in the formation of an extremely hygroscopic solid.
Phosphorous pentoxide is widely used as a dehydrating agent. On exposure to hydrolysis it forms phosphoric acid and releases heat.
To answer this question you must recall the oxides of phosphorus and their structures. ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$ is a dimer of ${{\text{P}}_2}{{\text{O}}_3}$.
Complete step by step solution:
${{\text{P}}_2}{{\text{O}}_3}$/ ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$ (phosphorus trioxide) is also known as phosphorous oxide or phosphorous anhydride because it is the anhydride of orthophosphoric acid. It is a soft white solid.
We know that since it is anhydride of an acid it will be acidic itself also and hydrolysis in water to form phosphorous acid. Phosphorus trioxide exists as a dimer and is written as ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$.
The structure of phosphorus trioxide dimer is,

From the structure, we can clearly see that each phosphorus atom is bonded to three oxygen atoms in a cyclic structure.
Thus, there are 12 ${\text{P - O}}$ bonds in ${{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}$.
The correct option is C.
Note:
Phosphorus forms two common oxides one of which is phosphorus trioxide.
The other common oxide of phosphorus is phosphorus pentoxide.
Phosphorus pentoxide has a molecular formula ${{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}$ and its common name is derived from its empirical formula,${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}$. It is a white crystalline solid. It is the anhydride of ortho phosphoric acid. It is a very powerful and useful desiccant and dehydrating agent.
Phosphorus pentoxide majorly crystallizes in four forms which are also known as polymorphs. The most common one is a metastable form, comprises molecules of ${{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}$. These molecules are held together in a hexagonal lattice. This form can be prepared by condensing the vapour of phosphorus pentoxide rapidly, resulting in the formation of an extremely hygroscopic solid.
Phosphorous pentoxide is widely used as a dehydrating agent. On exposure to hydrolysis it forms phosphoric acid and releases heat.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




