
The number of covalent bonds in ${{C}_{4}}{{H}_{7}}Br$, is:
(a)- 12
(b)- 10
(c)- 13
(d)- 11
Answer
569.4k+ views
Hint: The covalent bonds are formed when electrons are shared between two atoms, only when both the atoms contribute one-one electron. ${{C}_{4}}{{H}_{7}}Br$ is an alkene or specifically saying bromobutene by the replacement of one hydrogen by the bromine atom in butene.
Complete Solution :
- A bond is used to connect two atoms in a molecule. There are three types of bonds, i.e., ionic, covalent, and coordinate. An ionic bond is always formed between ions or we can say that one atom must be metal and the other must be non-metal, and there is the complete transference of electrons of one atom to the other.
- The covalent bonds are formed when electrons are shared between two atoms, only when both the atoms contribute one-one electron and it is formed between two nonmetals.
- The coordination bond is a special type of covalent formed between nonmetals by the complete transference of electrons of one atom to the other.
- In hydrocarbons, mostly all the bonds are covalent because all the atoms are non-metals. ${{C}_{4}}{{H}_{7}}Br$ is an alkene or specifically saying bromobutene by the replacement of one hydrogen by the bromine atom in butene. The open-chain structure of ${{C}_{4}}{{H}_{7}}Br$ is given below:
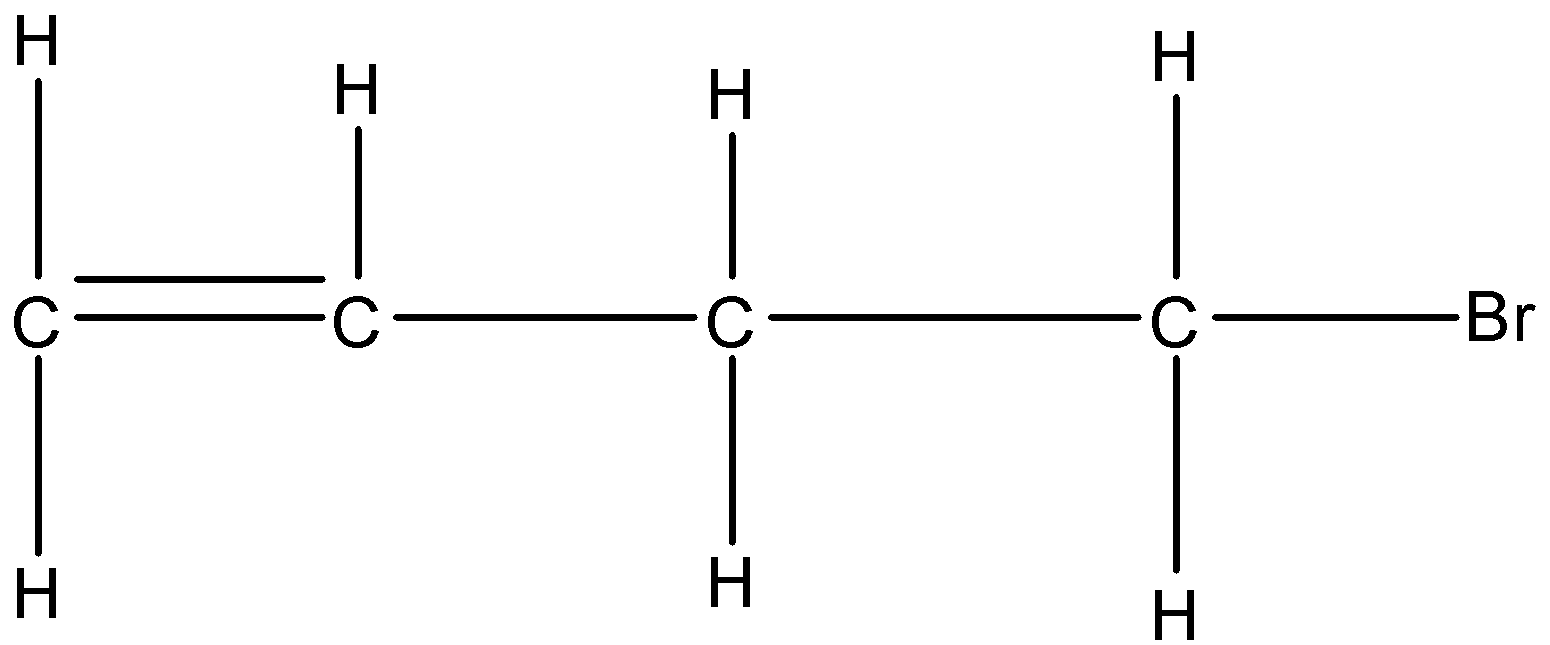
So, in this compound, there are 12 bonds and all the bonds are covalent because all the atoms are non-metals. Therefore, there are 12 covalent bonds in ${{C}_{4}}{{H}_{7}}Br$.
So, the correct answer is “Option A”.
Note: The covalent bond is a weaker bond than the ionic bond because the ionic bond is formed between ions that have mutual attractions, but in a covalent bond there are no such interactions seen. There are two types of a covalent bond, i.e, sigma and pi-bond.
Complete Solution :
- A bond is used to connect two atoms in a molecule. There are three types of bonds, i.e., ionic, covalent, and coordinate. An ionic bond is always formed between ions or we can say that one atom must be metal and the other must be non-metal, and there is the complete transference of electrons of one atom to the other.
- The covalent bonds are formed when electrons are shared between two atoms, only when both the atoms contribute one-one electron and it is formed between two nonmetals.
- The coordination bond is a special type of covalent formed between nonmetals by the complete transference of electrons of one atom to the other.
- In hydrocarbons, mostly all the bonds are covalent because all the atoms are non-metals. ${{C}_{4}}{{H}_{7}}Br$ is an alkene or specifically saying bromobutene by the replacement of one hydrogen by the bromine atom in butene. The open-chain structure of ${{C}_{4}}{{H}_{7}}Br$ is given below:

So, in this compound, there are 12 bonds and all the bonds are covalent because all the atoms are non-metals. Therefore, there are 12 covalent bonds in ${{C}_{4}}{{H}_{7}}Br$.
So, the correct answer is “Option A”.
Note: The covalent bond is a weaker bond than the ionic bond because the ionic bond is formed between ions that have mutual attractions, but in a covalent bond there are no such interactions seen. There are two types of a covalent bond, i.e, sigma and pi-bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




