
The number of alkenyl groups possible Structural Isomers) from ${C_4}{H_7}$ - are:
A. $7$
B. $5$
C. $3$
D. $8$
Answer
587.7k+ views
Hint: Isomers are chemical compounds which have the same chemical formula but different structure along with different physical properties. There can be chain isomerism, positional isomerism and functional group isomerism.
Step by step answer: An alkenyl group is a hydrocarbon group formed when an hydrogen group is removed from an alkene group. They are named after replacing ‘e’ from the alkene group by “yl”.
The structural isomers are the one in which the molecular formulae of the compounds are the same but they differ in their structural formulae. The structural Isomers are categorized into different sub-groups. Chain Isomers, Positional isomers, Functional group isomers. Chain Isomers are the one in which carbon chain arrangement is varied for the same molecular formulae. Positional Isomers are the one which have the same molecular formulae and same carbon skeleton structure but vary in the functional group position. The functional group isomers are the one which have the same molecular formula but different functional groups.
All the possible isomers of alkenyl groups are :
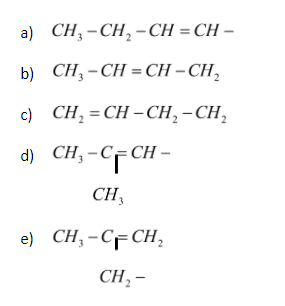
Hence, there are 5 different isomers which can be framed with the alkenyl group.
Therefore the correct option is (B).
Note: Isomers are different from Stereoisomers. Isomers are the one having the same molecular formula but different physical and chemical properties. Stereoisomers are the compounds having the same molecular formula but differ in the arrangements of the atoms in the three dimension space.They vary in the conformation and spatial arrangements of the molecule. Stereoisomers are of two different types (Cis/Trans) Isomers and Optical Isomers.
Step by step answer: An alkenyl group is a hydrocarbon group formed when an hydrogen group is removed from an alkene group. They are named after replacing ‘e’ from the alkene group by “yl”.

The structural isomers are the one in which the molecular formulae of the compounds are the same but they differ in their structural formulae. The structural Isomers are categorized into different sub-groups. Chain Isomers, Positional isomers, Functional group isomers. Chain Isomers are the one in which carbon chain arrangement is varied for the same molecular formulae. Positional Isomers are the one which have the same molecular formulae and same carbon skeleton structure but vary in the functional group position. The functional group isomers are the one which have the same molecular formula but different functional groups.
All the possible isomers of alkenyl groups are :
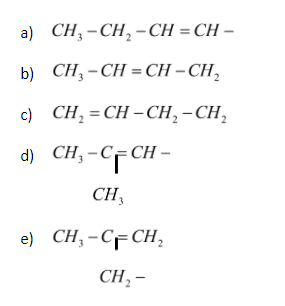
Hence, there are 5 different isomers which can be framed with the alkenyl group.
Therefore the correct option is (B).
Note: Isomers are different from Stereoisomers. Isomers are the one having the same molecular formula but different physical and chemical properties. Stereoisomers are the compounds having the same molecular formula but differ in the arrangements of the atoms in the three dimension space.They vary in the conformation and spatial arrangements of the molecule. Stereoisomers are of two different types (Cis/Trans) Isomers and Optical Isomers.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




