
The no. of P – O – P bond (s) in cyclic metaphosphoric acid is (are):
A. 0
B. 2
C. 3
D. 4
Answer
615.3k+ views
Hint- To find the number of bonds in cyclic metaphosphoric acid, refer to the structure of the given acid and then find the number of P – O – P bonds.
Complete answer:
We have been given to find the number of P – O – P bonds in the cyclic metaphosphoric acid.
So, let us first draw the structure of the given acid.
Refer the figure below to know the structure-
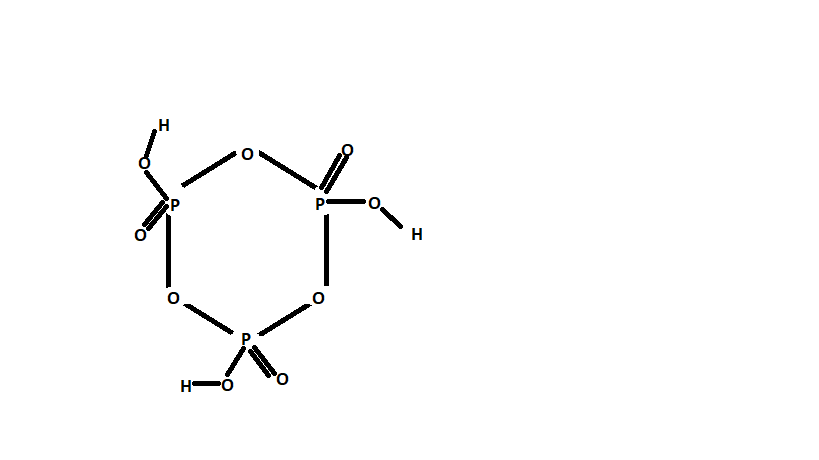
The figure shows the structure of cyclic meta phosphoric acid.
Now, as we can see that the above structure has P – O – P bond, P=O bond and P – O – H bonds.
So, if we count then the number of P – O – P bonds are three (See in the figure)
Hence, the number of P – O – P bonds in the structure of cyclic metaphosphoric acid is three.
Therefore, the correct option is C.
Note – Whenever it is asked to tell the number of bonds of any acid or compound then always first draw the structure of that compound and then as we can see the given structure has 3 P – O – P bonds, 3 P = O bonds and 3 P – O – H bonds.
Complete answer:
We have been given to find the number of P – O – P bonds in the cyclic metaphosphoric acid.
So, let us first draw the structure of the given acid.
Refer the figure below to know the structure-

The figure shows the structure of cyclic meta phosphoric acid.
Now, as we can see that the above structure has P – O – P bond, P=O bond and P – O – H bonds.
So, if we count then the number of P – O – P bonds are three (See in the figure)
Hence, the number of P – O – P bonds in the structure of cyclic metaphosphoric acid is three.
Therefore, the correct option is C.
Note – Whenever it is asked to tell the number of bonds of any acid or compound then always first draw the structure of that compound and then as we can see the given structure has 3 P – O – P bonds, 3 P = O bonds and 3 P – O – H bonds.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




