
The most direct malonic ester synthesis of the 3 – phenyl propanoic acid would involve the use of:
A. ${C_6}{H_5}C{H_2}C{H_2}C{H_2}CI$
B. ${C_6}{H_5}C{H_2}C{H_2}CI$
C. ${C_6}{H_5}C{H_2}CI$
D. $CIC{H_2}COOH$
Answer
589.5k+ views
Hint: The reaction of benzyl chloride with diethyl malonate in ethoxide/ethanol produces the alkylated product and the acidification hydrolyses the esters and the intermediate diacid decarboxylates to form the final product i.e.3 – phenyl propanoic acid.
Complete step by step answer:
Malonic ester is a reagent which is specifically used in a reaction which converts alkyl halides to carboxylic acids called the Malonic Ester Synthesis. Malonic ester synthesis is a synthetic procedure used to convert a compound that has the general structural formula 1 into a carboxylic acid that has the general structural formula 2.
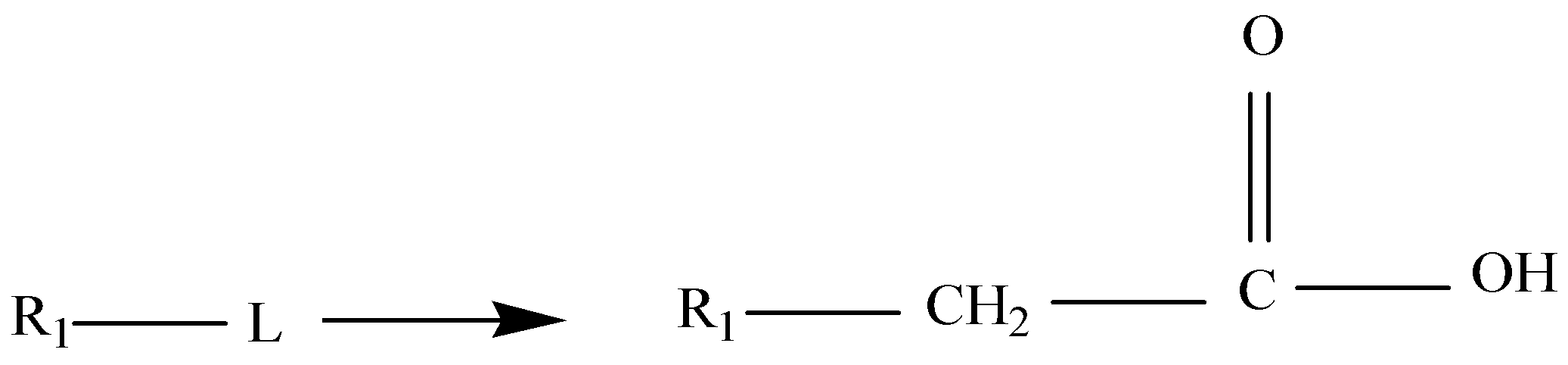
Where ${R_1}$ is an alkyl group and L is a leaving group. The group \[--C{H_2}C{O_2}H\] in 2 is contributed by a malonic ester, hence the term malonic ester synthesis.
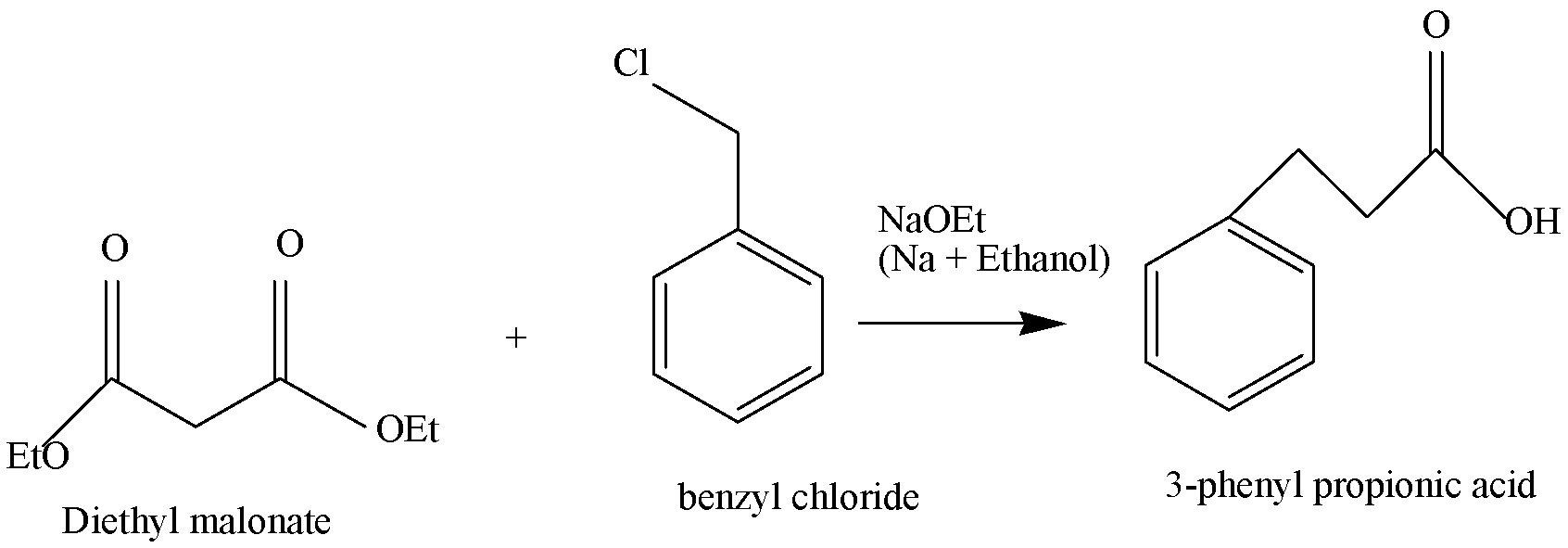
Firstly, freshly prepared sodium ethoxide reacts with diethyl malonate giving enolate ions which alkylate with the benzylated malonic ester. Reaction of benzyl chloride with diethyl malonate in ethoxide/ethanol yields the alkylated product. Acidification hydrolyses the esters and the intermediate diacid decarboxylates to form the final product, 3 – phenyl propanoic acid in the presence of a base like NaOH (in the above reaction).
So, the most direct malonic ester synthesis of the 3 – phenyl propanoic acid would involve the use of benzyl chloride i.e. ${C_6}{H_5}C{H_2}CI$ .
Therefore, the correct answer is option (C).
Note: One of the most valuable methods to prepare carboxylic acids makes use of ethyl malonate which is the malonic ester. 3 – phenyl propanoic acid is used as a flavouring agent, food additives, fragrance, spices etc. As it acts as a preservative.
Complete step by step answer:
Malonic ester is a reagent which is specifically used in a reaction which converts alkyl halides to carboxylic acids called the Malonic Ester Synthesis. Malonic ester synthesis is a synthetic procedure used to convert a compound that has the general structural formula 1 into a carboxylic acid that has the general structural formula 2.
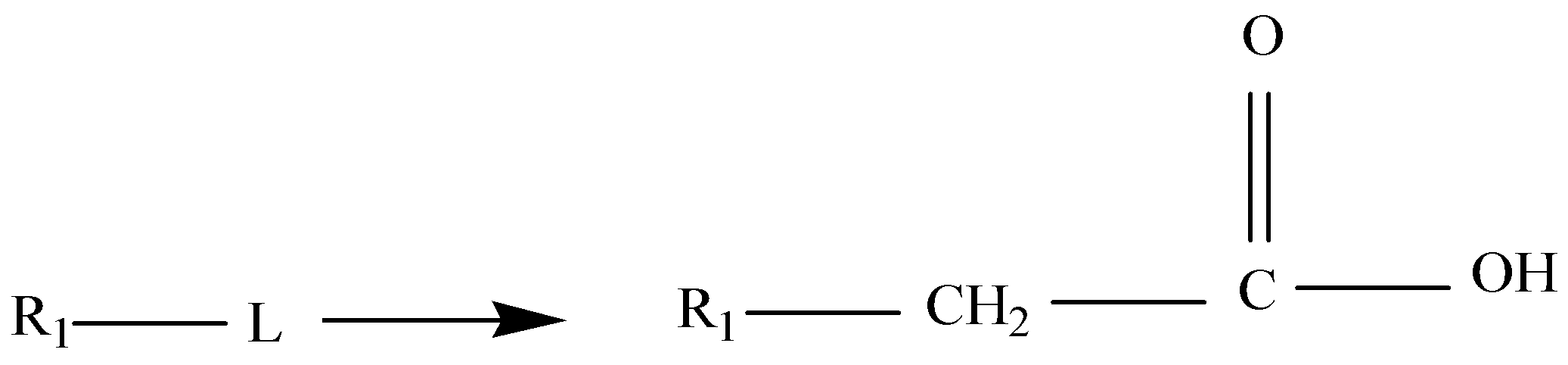
Where ${R_1}$ is an alkyl group and L is a leaving group. The group \[--C{H_2}C{O_2}H\] in 2 is contributed by a malonic ester, hence the term malonic ester synthesis.

Firstly, freshly prepared sodium ethoxide reacts with diethyl malonate giving enolate ions which alkylate with the benzylated malonic ester. Reaction of benzyl chloride with diethyl malonate in ethoxide/ethanol yields the alkylated product. Acidification hydrolyses the esters and the intermediate diacid decarboxylates to form the final product, 3 – phenyl propanoic acid in the presence of a base like NaOH (in the above reaction).
So, the most direct malonic ester synthesis of the 3 – phenyl propanoic acid would involve the use of benzyl chloride i.e. ${C_6}{H_5}C{H_2}CI$ .
Therefore, the correct answer is option (C).
Note: One of the most valuable methods to prepare carboxylic acids makes use of ethyl malonate which is the malonic ester. 3 – phenyl propanoic acid is used as a flavouring agent, food additives, fragrance, spices etc. As it acts as a preservative.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




